Inherited PURA Pathogenic Variant Associated With a Mild Neurodevelopmental Disorder
- PMID: 39131487
- PMCID: PMC11309559
- DOI: 10.1212/NXG.0000000000200181
Inherited PURA Pathogenic Variant Associated With a Mild Neurodevelopmental Disorder
Abstract
Objectives: Purine-rich element-binding protein alpha (PURA) regulates gene expression and is ubiquitously expressed with an enrichment in neural tissues. Pathogenic variants in PURA cause the neurodevelopmental disorder PURA syndrome that has a variable phenotype but typically comprises moderate-to-severe global developmental delay, intellectual disability, early-onset hypotonia and hypothermia, epilepsy, feeding difficulties, movement disorders, and subtle facial dysmorphism. Speech is reportedly absent in most, but the specific linguistic phenotype is not well described.
Methods: We used genome sequencing to identify a pathogenic gene variant as part of a study of children ascertained for severe primary speech disorder in the absence of moderate or severe ID.
Results: The novel PURA c.296G>T (p.Arg99Leu) pathogenic missense variant segregated in the female proband and her affected mother. The proband had dysarthria; phonological disorder; and severe receptive and expressive language impairment, borderline intellect, attention difficulties, oropharyngeal dysmotility, and dysmorphic facial features. Her mother had dysarthria, moderate receptive language impairment, and borderline intellect. Both the proband and her mother completed mainstream schooling with classroom support.
Discussion: This is the first inherited PURA pathogenic germline variant in over 600 unrelated families documented on ClinVar or reported in the literature. PURA testing should be considered in families with primary speech disorder and borderline intellectual disability, given the specific genetic counseling implications.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
I.E. Scheffer has served on scientific advisory boards for BioMarin, Chiesi, Eisai, Encoded Therapeutics, GlaxoSmithKline, Knopp Biosciences, Nutricia, Rogcon, Takeda Pharmaceuticals, UCB, Xenon Pharmaceuticals, Cerecin; has received speaker honoraria from GlaxoSmithKline, UCB, BioMarin, Biocodex, Chiesi, Liva Nova, Nutricia, Zuellig Pharma, Stoke Therapeutics and Eisai; has received funding for travel from UCB, Biocodex, GlaxoSmithKline, Biomarin, Encoded Therapeutics, Stoke Therapeutics and Eisai; has served as an investigator for Anavex Life Sciences, Cerevel Therapeutics, Eisai, Encoded Therapeutics, EpiMinder Inc, Epygenyx, ES-Therapeutics, GW Pharma, Marinus, Neurocrine BioSciences, Ovid Therapeutics, Takeda Pharmaceuticals, UCB, Ultragenyx, Xenon Pharmaceuticals, Zogenix and Zynerba; and has consulted for Care Beyond Diagnosis, Epilepsy Consortium, Atheneum Partners, Ovid Therapeutics, UCB, Zynerba Pharmaceuticals, BioMarin, Encoded Therapeutics and Biohaven Pharmaceuticals; and is a Non-Executive Director of Bellberry Ltd and a Director of the Australian Academy of Health and Medical Sciences and the Australian Council of Learned Academies Limited. She may accrue future revenue on pending patent WO61/010176 (filed: 2008): Therapeutic Compound; has a patent for SCN1A testing held by Bionomics Inc and licensed to various diagnostic companies; has a patent molecular diagnostic/theranostic target for benign familial infantile epilepsy (BFIE) [PRRT2] 2011904493 and 2012900190 and PCT/AU2012/001321 (TECH ID:2012-009). The remaining authors declare no competing interests. Go to Neurology.org/NG for full disclosures.
Figures
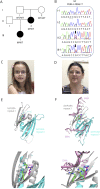
Similar articles
-
Expanding the phenotype of PURA-related neurodevelopmental disorder: a close differential diagnosis of infantile hypotonia with psychomotor retardation and characteristic facies.Clin Dysmorphol. 2021 Jan;30(1):1-5. doi: 10.1097/MCD.0000000000000360. Clin Dysmorphol. 2021. PMID: 33229923 Free PMC article.
-
Expanding the clinical phenotype and genetic spectrum of PURA-related neurodevelopmental disorders.Brain Dev. 2021 Oct;43(9):912-918. doi: 10.1016/j.braindev.2021.05.009. Epub 2021 Jun 8. Brain Dev. 2021. PMID: 34116881
-
PURA syndrome: clinical delineation and genotype-phenotype study in 32 individuals with review of published literature.J Med Genet. 2018 Feb;55(2):104-113. doi: 10.1136/jmedgenet-2017-104946. Epub 2017 Nov 2. J Med Genet. 2018. PMID: 29097605 Free PMC article. Review.
-
Expanding the PURA syndrome phenotype: A child with the recurrent PURA p.(Phe233del) pathogenic variant showing similarities with cutis laxa.Mol Genet Genomic Med. 2021 Jan;9(1):e1562. doi: 10.1002/mgg3.1562. Epub 2020 Dec 4. Mol Genet Genomic Med. 2021. PMID: 33275834 Free PMC article.
-
A new case with the recurrent PURA p.(Phe233del) pathogenic variant: Expansion of the phenotype and review of the literature.Int J Dev Neurosci. 2023 Jun;83(4):383-395. doi: 10.1002/jdn.10266. Epub 2023 May 19. Int J Dev Neurosci. 2023. PMID: 37204304 Review.
Cited by
-
PURA syndrome-a genetic cause of a neurodevelopmental disorder-case report.Front Pediatr. 2025 Jul 10;13:1607213. doi: 10.3389/fped.2025.1607213. eCollection 2025. Front Pediatr. 2025. PMID: 40708900 Free PMC article.
-
Genotype-phenotype variations in PURA syndrome: Asian and non-Asian perspectives from a systematic review.Orphanet J Rare Dis. 2025 Jul 25;20(1):376. doi: 10.1186/s13023-025-03908-9. Orphanet J Rare Dis. 2025. PMID: 40713824 Free PMC article.
References
-
- NCBI ClinVar. Accessed July 30, 2023. ncbi.nlm.nih.gov/clinvar/
LinkOut - more resources
Full Text Sources
