Synthesis of novel piperazine-based bis(thiazole)(1,3,4-thiadiazole) hybrids as anti-cancer agents through caspase-dependent apoptosis
- PMID: 39131497
- PMCID: PMC11310838
- DOI: 10.1039/d4ra05091f
Synthesis of novel piperazine-based bis(thiazole)(1,3,4-thiadiazole) hybrids as anti-cancer agents through caspase-dependent apoptosis
Abstract
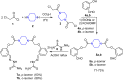
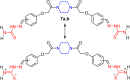
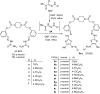
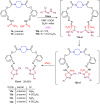
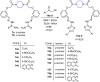
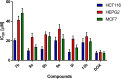
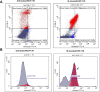
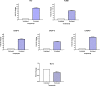
A series of novel piperazine-based bis(thiazoles) 13a-d were synthesized in moderate to good yields via reaction of the bis(thiosemicarbazones) 7a, b with an assortment of C-acetyl-N-aryl-hydrazonoyl chlorides 8a-f. Similar treatment of the bis(thiosemicarbazone) 7a, b with C-aryl-N-phenylhydrazonoyl chlorides 10a, b afforded the expected bis(thiadiazole) based piperazine products 13b-d in reasonable yields. Cyclization of 7a, b with two equivalents of α-haloketones 14a-d led to the production of the corresponding bis(4-arylthiazol)piperazine derivatives 15a-h in good yields. The structures of the synthesized compounds were confirmed from elemental and spectral data (FTIR, MALDI-TOF, 1H, and 13C NMR). The cytotoxicity of the new compounds was screened against hepatoblastoma (HepG2), human colorectal carcinoma (HCT 116), breast cancer (MCF-7), and Human Dermal Fibroblasts (HDF). Interestingly, all compounds showed promising cytotoxicity against most of the cell lines. Interestingly, compounds 7b, 9a, and 9i exhibited IC50 values of 3.5, 12.1, and 1.2 nM, respectively, causing inhibition of 89.7%, 83.7%, and 97.5%, compared to Erlotinib (IC50 = 1.3 nM, 97.8% inhibition). Compound 9i dramatically induced apoptotic cell death by 4.16-fold and necrosis cell death by 4.79-fold. Compound 9i upregulated the apoptosis-related genes and downregulated the Bcl-2 as an anti-apoptotic gene. Accordingly, the most promising EGFR-targeted chemotherapeutic agent to treat colon cancer was found to be compound 9i.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Novel Bis-Thiazole Derivatives: Synthesis and Potential Cytotoxic Activity Through Apoptosis With Molecular Docking Approaches.Front Chem. 2021 Aug 12;9:694870. doi: 10.3389/fchem.2021.694870. eCollection 2021. Front Chem. 2021. PMID: 34458233 Free PMC article.
-
Synthesis of novel mono- and bis-pyrazolylthiazole derivatives as anti-liver cancer agents through EGFR/HER2 target inhibition.BMC Chem. 2023 Jun 8;17(1):51. doi: 10.1186/s13065-023-00921-6. BMC Chem. 2023. PMID: 37291635 Free PMC article.
-
Design and synthesis of new bis(1,2,4-triazolo[3,4-b][1,3,4]thiadiazines) and bis((quinoxalin-2-yl)phenoxy)alkanes as anti-breast cancer agents through dual PARP-1 and EGFR targets inhibition.RSC Adv. 2022 Aug 19;12(36):23644-23660. doi: 10.1039/d2ra03549a. eCollection 2022 Aug 16. RSC Adv. 2022. PMID: 36090415 Free PMC article.
-
Novel bis-amide-based bis-thiazoles as Anti-colorectal Cancer Agents Through Bcl-2 Inhibition: Synthesis, In Vitro, and In Vivo studies.Anticancer Agents Med Chem. 2023;23(3):328-345. doi: 10.2174/1871520622666220615140239. Anticancer Agents Med Chem. 2023. PMID: 35708084
-
Synthesis, characterization, molecular docking, pharmacokinetics, and molecular dynamics of new bis-thiazoles based on bis-thiosemicarbazone as anti-coxsackievirus.Sci Rep. 2024 Nov 26;14(1):29378. doi: 10.1038/s41598-024-80753-z. Sci Rep. 2024. PMID: 39592765 Free PMC article.
Cited by
-
Unraveling Structural and Anticancer Properties of Pyridine-Oxadiazole Derivatives: Single-Crystal XRD, Hirshfeld Analysis, and Cytotoxicity against A549 Cells.ACS Omega. 2025 Jun 1;10(22):23549-23562. doi: 10.1021/acsomega.5c02152. eCollection 2025 Jun 10. ACS Omega. 2025. PMID: 40521477 Free PMC article.
-
Recent progress in therapeutic applications of fluorinated five-membered heterocycles and their benzo-fused systems.RSC Adv. 2024 Oct 25;14(46):33864-33905. doi: 10.1039/d4ra05697c. eCollection 2024 Oct 23. RSC Adv. 2024. PMID: 39463482 Free PMC article. Review.
-
Recent advances on anticancer activity of benzodiazine heterocycles through kinase inhibition.RSC Adv. 2025 Feb 19;15(7):5597-5638. doi: 10.1039/d4ra08134j. eCollection 2025 Feb 13. RSC Adv. 2025. PMID: 39974315 Free PMC article. Review.
-
Synthesis of some new pyrimidine-based pyrene/benzochromene hybrids as EGFR kinase inhibitors in HCT-116 cancer cells through apoptosis.RSC Adv. 2025 Aug 28;15(37):30683-30696. doi: 10.1039/d5ra03611a. eCollection 2025 Aug 22. RSC Adv. 2025. PMID: 40895751 Free PMC article.
References
-
- Gettys K. E. Ye Z. Dai M. Synthesis. 2017;49:2589–2604.
-
- Guo C. C. Li H.-P. Zhang X.-B. Bioorg. Med. Chem. 2003;11:1745–1751. - PubMed
-
- Guo C. C. Tong R.-B. Li K.-L. Bioorg. Med. Chem. 2004;12:2469–2475. - PubMed
-
- Vitaku E. Smith D. T. Njardarson J. T. J. Med. Chem. 2014;57:10257–10274. - PubMed
-
- Rizwan M. Noreen S. Asim S. Liaqat Z. Shaheen M. Ibrahim H. Chem. Inorg. Mater. 2024;2:100041.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

