Bruton tyrosine kinase inhibitor-related atrial fibrillation and its implications in the treatment of B-cell lymphoma
- PMID: 39131702
- PMCID: PMC11310794
- DOI: 10.3389/fcvm.2024.1408983
Bruton tyrosine kinase inhibitor-related atrial fibrillation and its implications in the treatment of B-cell lymphoma
Abstract
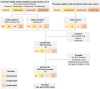
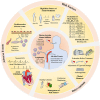
Adverse events of atrial fibrillation (AF) have been commonly reported in lymphoma patients in treating Bruton's tyrosine kinase inhibitors (BTKi). The incidence rate of AF can vary depending on the specific types of BTKi and the patient population. Totally 45 published studies have revealed that the overall incidence rate of AF is 5% (95% CI 4%-7%). By performing a subtype single-rate analysis, the second-generation BTKi shows a lower AF incidence rate and lower cardiovascular toxicity. In the subtype single-rate analysis, we conclude the different AF incidence rates of Ibrutinib (10%, 95% CI 7%-13%), Acalabrutinib (4%, 95% CI 1%-6%), Orelabrutinib (0%, 95% CI 0%-1%), and Zanubrutinib (0%, 95% CI 0%-1%). The comprehensive analysis of AF inspires us to better predict and manage AF and other cardiovascular events in treating lymphoma. Meticulous evaluation, collaboration between cardiologists and hematologists, and discovery of new biomarkers are essential for its management.
Keywords: Bruton tyrosine kinase inhibitors; Ibrutinib; atrial fibrillation; cardiovascular toxicity; lymphoma.
© 2024 Du, Chen, Gu, Wang and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

