Outcomes of Continuation vs Discontinuation of Adalimumab Therapy During Third Trimester of Pregnancy in Inflammatory Bowel Disease
- PMID: 39131851
- PMCID: PMC11307739
- DOI: 10.1016/j.gastha.2022.04.009
Outcomes of Continuation vs Discontinuation of Adalimumab Therapy During Third Trimester of Pregnancy in Inflammatory Bowel Disease
Abstract
Background and aims: Adalimumab (ADA) transport across the placenta increases with gestational age advances. We evaluated child-mother health outcomes related to the timing of the last ADA dose before delivery.
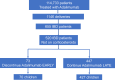
Methods: Using IBM MarketScan data, we collected records for all children exposed to ADA during intrauterine life. We compared milestone achievements, congenital malformations, and respiratory infections rates in children from mothers of 2 groups: (1) a late ADA group, which continued therapy until 90 days or fewer before delivery; and (2) an early ADA group, which discontinued therapy more than 90 days before delivery. We also assessed the risk of flaring for mothers in the early group.
Results: There were no significant differences in growth (P = .48), developmental delays (P = .25), or congenital malformations (P = .61) in the 427 children of the late group vs 70 children of early ADA group. Continuing ADA late in pregnancy did not increase the respiratory infection rate (P = .38). No differences occurred between groups in cesarean and premature delivery, intrauterine growth retardation, and stillbirths. ADA discontinuation was the only predictor of flaring in the third trimester of pregnancy (odds ratio = 6.04, 95% confidence interval 2.66-13.7). In the late group, mothers' risk of flaring decreased (16/447 vs 13/73, P < .001). Mothers with active disease were more likely to deliver prematurely vs mothers with quiet disease (6/29 vs 31/491, P = .003).
Conclusion: Continuation of ADA in pregnancy close to delivery is of low risk for children. Early discontinuation, however, increases the risk of flaring in mothers and the likelihood of premature deliveries.
Keywords: Adalimumab; Congenital Malformation; Inflammatory Bowel Disease; Pregnancy.
© 2022 Published by Elsevier Inc. on behalf of the AGA Institute.
Figures
Similar articles
-
Early Discontinuation of Infliximab in Pregnant Women With Inflammatory Bowel Disease.Inflamm Bowel Dis. 2020 Jun 18;26(7):1110-1117. doi: 10.1093/ibd/izz250. Inflamm Bowel Dis. 2020. PMID: 31670762
-
Screening for small for gestational age infants in early vs late third-trimester ultrasonography: a randomized trial.Am J Obstet Gynecol MFM. 2023 Nov;5(11):101162. doi: 10.1016/j.ajogmf.2023.101162. Epub 2023 Sep 15. Am J Obstet Gynecol MFM. 2023. PMID: 37717697 Clinical Trial.
-
Adalimumab discontinuation in patients with early rheumatoid arthritis who were initially treated with methotrexate alone or in combination with adalimumab: 1 year outcomes of the HOPEFUL-2 study.RMD Open. 2016 Feb 18;2(1):e000189. doi: 10.1136/rmdopen-2015-000189. eCollection 2016. RMD Open. 2016. PMID: 26925252 Free PMC article.
-
Maternal and neonatal outcomes after bariatric surgery; a systematic review and meta-analysis: do the benefits outweigh the risks?Am J Obstet Gynecol. 2018 Jun;218(6):573-580. doi: 10.1016/j.ajog.2018.02.003. Epub 2018 Feb 15. Am J Obstet Gynecol. 2018. PMID: 29454871
-
Anti-Drug Antibody Formation Against Biologic Agents in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis.BioDrugs. 2021 Nov;35(6):715-733. doi: 10.1007/s40259-021-00507-5. Epub 2021 Nov 19. BioDrugs. 2021. PMID: 34797516 Free PMC article.
Cited by
-
Use of Biologics During Pregnancy Among Patients With Autoimmune Conditions.JAMA Netw Open. 2025 May 1;8(5):e2510504. doi: 10.1001/jamanetworkopen.2025.10504. JAMA Netw Open. 2025. PMID: 40372753 Free PMC article.
-
The Role of Adalimumab in Recurrent Pregnancy Loss Due Immune Dysregulation: A Case Series Report.J Obstet Gynaecol India. 2025 Apr;75(Suppl 1):644-646. doi: 10.1007/s13224-024-02083-4. Epub 2025 Feb 1. J Obstet Gynaecol India. 2025. PMID: 40390909
-
A Guide to the Management of Hidradenitis Suppurativa in Pregnancy and Lactation.Am J Clin Dermatol. 2025 May;26(3):345-360. doi: 10.1007/s40257-025-00935-x. Epub 2025 Mar 25. Am J Clin Dermatol. 2025. PMID: 40131719 Free PMC article. Review.
References
-
- Kim M.A., Kim Y.H., Chun J., et al. The influence of disease activity on pregnancy outcomes in women with inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2021;15:719–732. - PubMed
-
- Aboubakr A., Riggs A.R., Jimenez D., et al. Identifying patient priorities for preconception and pregnancy counseling in IBD. Dig Dis Sci. 2021;66:1829–1835. - PubMed
-
- Van der Woude C.J., Ardizzone S., Bengtson M.B., et al. European Crohn's and Colitis Organization (ECCO), the second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis. 2015;9:107–124. - PubMed
-
- Mahadevan U., Robinson C., Bernasko N., et al. Inflammatory bowel disease in pregnancy clinical care pathway: a report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology. 2019;156:1508–1524. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials