Early and short-term use of proprotein convertase anti-subtilisin-kexin type 9 inhibitors on coronary plaque stability in acute coronary syndrome
- PMID: 39131906
- PMCID: PMC11316204
- DOI: 10.1093/ehjopen/oeae055
Early and short-term use of proprotein convertase anti-subtilisin-kexin type 9 inhibitors on coronary plaque stability in acute coronary syndrome
Abstract
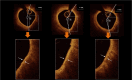
Aims: Proprotein convertase anti-subtilisin-kexin type 9 inhibitors (PCSK9Is) improve plaque volume and composition and reduce major adverse coronary events in chronic coronary artery disease. We evaluated the effects of the short-term use of PCSK9Is on coronary plaque stability in patients with acute coronary syndrome (ACS) using optical coherence tomography (OCT).
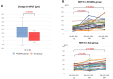
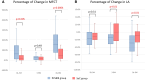
Methods and results: This is a multicentre, open-label randomized controlled trial. The enrolled 80 subjects met the inclusion criteria. Of these, 52 patients (age 60 ± 11 years, 38 men, 14 women) with ST-elevated ACS had undergone successful primary percutaneous coronary intervention with LDL-cholesterol (LDL-C) levels > 70 mg/dL while receiving high-intensity statins. Participants were randomly assigned to the PCSK9I group (evolocumab 420 mg for 3 months, n = 29) or the standard of care (SoC) group (n = 23). Optical coherence tomography was performed at baseline (BL) and 3 and 9 months after randomization to assess lipid-rich plaques in non-culprit lesions. The change in the minimum fibrous cap thickness (MFCT) from BL to 9 months was the primary endpoint. The percentage change in LDL-C levels from BL to 3 months was significantly greater in the PCSK9I group (-67.8 ± 21.5% in the PCSK9I group vs. -16.3 ± 21.8% in the SoC group; P < 0.0001), and the difference between the two groups disappeared from BL to 9 months (-20.0 ± 37.8% in the PCSK9I group vs. -6.7 ± 34.2% in the SoC group; P = 0.20). The changes in MFCT from BL to 9 months were significantly greater in the PCSK9I group, even after PCSK9I discontinuation {100 μm [interquartile range (IQR): 45-180 μm] vs. 50 μm [IQR: 0-110 μm]; P = 0.032}.
Conclusion: Combination treatment with PCSK9Is and statins resulted in more marked plaque stabilization after ACS than SoC alone, and this effect persisted for 6 months after PCSK9I discontinuation.
Registration: Adage-Joto study, UMIN ID No. 26516.
Keywords: Acute coronary syndrome; LDL-cholesterol; Optical coherence tomography; PCSK9 inhibitor; Statin.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: none declared.
Figures


References
-
- Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators . Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–1504. - PubMed
-
- Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR; FOURIER Steering Committee and Investigators . Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722. - PubMed
-
- Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby JF, Tricoci P, White HD, Zeiher AM; ODYSSEY OUTCOMES Committees and Investigators . Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018;379:2097–2107. - PubMed
-
- Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJ, Koenig W, Somaratne R, Kassahun H, Yang J, Wasserman SM, Scott R, Ungi I, Podolec J, Ophuis AO, Cornel JH, Borgman M, Brennan DM, Nissen SE. Effect of evolocumab on progression of coronary disease in statin-treated patients. The GLAGOV randomized clinical trial. JAMA 2016;316:2373–2384. - PubMed
-
- Nicholls SJ, Kataoka Y, Nissen SE, Prati F, Windecker S, Puri R, Hucko T, Aradi D, Herrman JR, Hermanides RS, Wang B, Wang H, Butters J, Di Giovanni G, Jones S, Pompili G, Psaltis PJ. Effect of evolocumab on coronary plaque phenotype and burden in statin-treated patients following myocardial infarction. JACC Cardiovasc Imaging 2022;15:1308–1321. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

