Fluorescein Angiography Parameters in Premature Neonates
- PMID: 39132023
- PMCID: PMC11315194
- DOI: 10.1016/j.xops.2024.100561
Fluorescein Angiography Parameters in Premature Neonates
Abstract
Purpose: To describe fluorescein angiography (FA) parameters observed in premature neonates with retinopathy of prematurity (ROP).
Design: Retrospective case series.
Subjects: Patients with ROP who underwent FA imaging using Retcam at Holtz Children's Hospital from November 2014 to October 2022.
Methods: Fluorescein angiography images of the included patients were analyzed with a focus on the timing of angiography phases, including choroidal flush, retinal, and recirculation phases. Gestational age, birth weight (BW), age at imaging, treatment choice, and any FA complications were documented.
Main outcome measures: Dose of fluorescein administered, onset and duration of each angiography phase, and FA findings in ROP-treated patients.
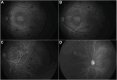
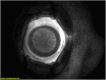
Results: A total of 72 images of 72 eyes were reviewed. Image quality was deemed suitable for inclusion in 64 eyes (88.9%) of 43 patients. The mean gestational age and BW at birth were 24.4 ± 1.9 weeks and 607.8 ± 141.3 g, respectively. The mean postmenstrual age at FA imaging was 50.5 ± 40.8 weeks. All eyes (100%) received treatment with intravitreal injection of anti-VEGF at a mean age of 35.5 ± 2.4 weeks. The onset and duration of angiography phases were relatively variable within the cohort. Choroidal flush occurred at a mean time of 12.2 seconds (range: 6-22 seconds). A subsequent retinal phase was documented at a mean time of 11.96 seconds (range: 3-22 seconds). Recirculation phase was complete at an average time of 2.15 minutes (range: 1-5.45 minutes) postfluorescein injection. None of patients developed allergic reactions to fluorescein injection, such as rash, respiratory distress, tachycardia, fever, or local injection site reactions.
Conclusions: Angiographic phases on FA in preterm infants with ROP are variable and may occur earlier than the established references for adults.
Financial disclosures: Proprietary or commercial disclosure may be found in the Footnotes and Disclosures at the end of this article.
Keywords: Fluorescein angiography; Neonates; Retinopathy of prematurity.
© 2024 Published by Elsevier Inc. on behalf of the American Academy of Ophthalmology.
Figures
References
-
- Vander J.F., Handa J., McNamara J.A., et al. Early treatment of posterior retinopathy of prematurity: a controlled trial. Ophthalmology. 1997;104:1731–1735. discussion 1735. - PubMed
-
- Early Treatment for Retinopathy of Prematurity Cooperative Group Revised indications for the treatment of retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–1696. - PubMed
-
- Kychenthal A., Dorta P., Katz X. Zone I retinopathy of prematurity: clinical characteristics and treatment outcomes. Retina. 2006;26:S11–S15. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

