A Meta-analysis of Standard Versus Ultrasound-Assisted Catheter-Directed Thrombolysis in the Management of Acute Pulmonary Embolism
- PMID: 39132529
- PMCID: PMC11308803
- DOI: 10.1016/j.jscai.2022.100514
A Meta-analysis of Standard Versus Ultrasound-Assisted Catheter-Directed Thrombolysis in the Management of Acute Pulmonary Embolism
Abstract
Background: Standard catheter-directed thrombolysis (SCDT) harnesses the therapeutic benefit of systemic thrombolytics while minimizing bleeding complications in patients presenting with pulmonary embolism (PE). Ultrasound-assisted catheter-directed thrombolysis (USAT) theoretically improves upon SCDT by disrupting fibrin and increasing the surface area exposed to thrombolytic agent. However, it is unclear if this translates into improved outcomes.
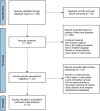
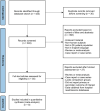
Methods: A systematic search of prior publications comparing SCDT and USAT in patients with intermediate or high-risk PE was conducted. Primary outcomes of interest were bleeding events, ICU and hospital length of stay. Secondary outcomes included changes in pulmonary artery systolic pressure (PASP), mean pulmonary artery pressure (mPAP), and right ventricle to left ventricle diameter (RV/LV) ratio. Studies that lacked comparison groups were excluded. Bias assessments were performed using the Cochrane tools for randomized and nonrandomized studies. Data was collated utilizing the Cochrane Review Manager software, and all analyses assumed random effects.
Results: Our search yielded 7 observational studies and 1 randomized control trial. The studies included a total of 543 patients who underwent either SCDT (n = 273) or USAT (n = 270) for intermediate or high-risk PE. The synthesized analysis showed no significant differences in bleeding between the groups. There were no differences in ICU or hospital lengths of stay, changes in PASP, or mPAP. Reductions in RV/LV ratio were greater with SCDT (mean difference, -0.16; 95% CI, -0.27 to -0.06; P = .003).
Conclusions: In comparison to SCDT, USAT did not result in improved clinical or hemodynamic outcomes in patients presenting with PE. Results were limited by heterogeneity among the included studies.
Keywords: catheters; meta-analysis; pulmonary embolism; thrombolysis; ultrasound.
© 2022 The Author(s).
Figures










References
-
- Jerjes-Sanchez C., Ramirez-Rivera A., de Lourdes Garcia M., et al. Streptokinase and heparin versus heparin alone in massive pulmonary embolism: a randomized controlled trial. J Thromb Thrombolysis. 1995;2(3):227–229. - PubMed
-
- Meyer G., Vicaut E., Danays T., et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370(15):1402–1411. - PubMed
-
- Francis C.W., Suchkova V.N. Ultrasound and thrombolysis. Vasc Med. 2001;6(3):181–187. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

