Sex-specific Long-term Outcomes of Watchman Left Atrial Appendage Closure for Stroke Prevention in Atrial Fibrillation
- PMID: 39132535
- PMCID: PMC11307581
- DOI: 10.1016/j.jscai.2022.100541
Sex-specific Long-term Outcomes of Watchman Left Atrial Appendage Closure for Stroke Prevention in Atrial Fibrillation
Abstract
Background: A recent analysis of a large registry showed differences in periprocedural outcomes of the Watchman left atrial appendage closure device in males compared with females. The objective of our study was to investigate the 5-year event rate in males and females enrolled in the Watchman device premarket clinical studies submitted for US Food and Drug Administration review.
Methods: We conducted a patient-level meta-analysis of 2256 patients from 4 studies: the PROTECT AF (Embolic Protection in Patients with Atrial Fibrillation) and PREVAIL (Prospective Randomized Evaluation of the Watchman Left Atrial Appendage Closure Device in Patients with Atrial Fibrillation vs Long-Term Warfarin Therapy) randomized controlled trials and their continued-access registries-CAP1 (Continued Access to PROTECT AF) and CAP2 (Continued Access to PREVAIL). The outcomes evaluated were ischemic stroke (IS), IS/systemic embolism, hemorrhagic stroke (HS), and all-cause mortality. Mixed-effects Cox regression models and statistical testing for treatment-by-sex interaction were used to compare left atrial appendage closure vs warfarin in males and females. Hazard ratios adjusted (aHRs) for CHADS2 scores were generated using the same model with CHADS2 score as a covariate. Time-to-event end points were evaluated using the Kaplan-Meier method and log-rank test.
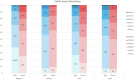
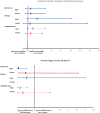
Results: For Watchman vs warfarin in the 2 randomized controlled trials, there was no significant interaction between sex and treatment for IS, IS/systemic embolism, HS, and all-cause mortality (P > .05); both males and females in the Watchman group had a lower aHR for HS than that in the warfarin group, which was statistically significant for males (aHR, 0.163; 95% CI, 0.045-0.593). In addition, there were no differences in outcomes between females and males treated with the Watchman device when pooling all studies.
Conclusions: These data suggest that sex does not significantly affect the long-term safety and effectiveness of the Watchman device in patients with nonvalvular atrial fibrillation; however, further studies are needed.
Keywords: Watchman; atrial fibrillation; females; left atrial appendage closure; meta-analysis.
Figures



References
-
- Atrial Fibrillation. Centers for Disease Control and Prevention. https://www.cdc.gov/heartdisease/atrial_fibrillation.htm
-
- Wolf P.A., Abbott R.D., Kannel W.B. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–988. - PubMed
-
- Holmes D.R., Reddy V.Y., Turi Z.G., et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374(9689):534–542. - PubMed
-
- Reddy V.Y., Doshi S.K., Kar S., et al. 5-year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol. 2017;70(24):2964–2975. - PubMed
-
- Reddy V.Y., Sievert H., Halperin J., et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312(19):1988–1998. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

