Hemodynamic Monitoring of Pediatric Patients With Heart Failure and Pulmonary Hypertension Using CardioMEMS
- PMID: 39132597
- PMCID: PMC11307819
- DOI: 10.1016/j.jscai.2024.101933
Hemodynamic Monitoring of Pediatric Patients With Heart Failure and Pulmonary Hypertension Using CardioMEMS
Abstract
Background: The CardioMEMS is an implantable device for hemodynamic monitoring approved by the US Food and Drug Administration for adult patients with heart failure. It has been used in the adult population without structural heart disease and with congenital heart diseases, but we do not have data in the pediatric population.
Methods: We report the initial single-center experience of the CardioMEMS implantation in children. Feasibility of device implantation, procedural outcomes, and clinical utility in the pediatric population were evaluated.
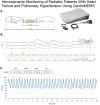
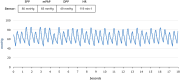
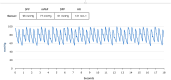
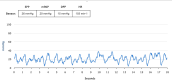
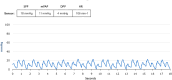
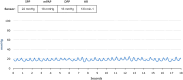
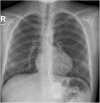
Results: The CardioMEMS device was implanted without technical complications in 8 pediatric patients (mean age 7 years and mean weight 27.9 kg) with pulmonary hypertension (6/8, 75%) and heart failure (2/8, 25%). The device was delivered via femoral access in 7 (85%) patients and implanted in the left pulmonary artery in 7 (85%). The noninvasive recording of pulmonary pressures in patients with pulmonary hypertension allowed the monitoring of the evolution of mean pulmonary artery pressure, intensifying vasodilator treatment, and avoiding control cardiac catheterizations. In patients with heart failure, pulmonary hemodynamic monitoring guided the decongestive treatment prior to heart transplantation.
Conclusions: The implantation of CardioMEMS in the pediatric population is a feasible procedure that allows the noninvasive hemodynamic monitoring of patients with heart failure and pulmonary hypertension. Its implementation in selected patients aids in outpatient follow-up and therapeutic management of patients with complex cardiac conditions, avoiding invasive procedures that require hospitalization. Further large-scale studies in the pediatric population are recommended.
Keywords: CardioMEMS; children; heart failure; implantable hemodynamic device; pulmonary hypertension; remote monitoring.
© 2024 The Author(s).
Figures

References
LinkOut - more resources
Full Text Sources

