PEERLESS II: A Randomized Controlled Trial of Large-Bore Thrombectomy Versus Anticoagulation in Intermediate-Risk Pulmonary Embolism
- PMID: 39132600
- PMCID: PMC11308785
- DOI: 10.1016/j.jscai.2024.101982
PEERLESS II: A Randomized Controlled Trial of Large-Bore Thrombectomy Versus Anticoagulation in Intermediate-Risk Pulmonary Embolism
Abstract
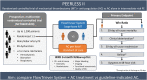
Background: Anticoagulation (AC) is the guideline-recommended treatment for intermediate-risk pulmonary embolism (PE); however, it remains unclear whether mechanical thrombectomy provides benefit over AC alone. The PEERLESS II study aims to evaluate outcomes in intermediate-risk PE patients randomized to treatment with large-bore mechanical thrombectomy and AC vs AC alone.
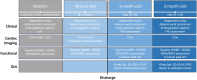
Methods: PEERLESS II is an international randomized controlled trial enrolling up to 1200 patients with intermediate-risk PE and additional clinical risk factors from up to 100 sites. Treatment is randomized 1:1 to large-bore mechanical thrombectomy with the FlowTriever System (Inari Medical) and AC or AC alone. Outcomes will be evaluated for up to 3 months, with safety events independently adjudicated. The primary end point is a hierarchical composite win ratio of (1) all-cause mortality by 30 days, (2) clinical deterioration (earlier of discharge or 30 days), (3) all-cause hospital readmission by 30 days, (4) bailout therapy (earlier of discharge or 30 days), and (5) Modified Medical Research Council (mMRC) dyspnea score of ≥1 at the 48-hour visit. Secondary end points include all-cause and PE-related mortality (30-day and 90-day), all-cause and PE-related readmission (30-day and 90-day), major bleeding (30-day and 90-day), clinical deterioration (earlier of discharge or 30 days), bailout (earlier of discharge or 30 days), right ventricle-to-left ventricle diameter ratio (48-hour visit), mMRC dyspnea score (48-hour, 1-month, and 3-month visits), quality of life using Pulmonary Embolism Quality of Life and EuroQol-5 Dimensions-5 Levels (1-month and 3-month visits), 6-minute walk distance (1-month visit), and post-PE impairment diagnosis (3-month visit).
Conclusions: PEERLESS II will inform the understanding of mechanical thrombectomy treatment for intermediate-risk PE and provide evidence for consideration in future treatment guidelines.
Keywords: anticoagulation; mechanical thrombectomy; pulmonary embolism; randomized controlled trial.
© 2024 The Author(s).
Conflict of interest statement
Jay Giri reports consulting fees, speaking fees, and research funds to the institution from Inari Medical and Boston Scientific and equity in Endovascular Engineering. Felix Mahfoud is supported by Deutsche Gesellschaft für Kardiologie (DGK), Deutsche Forschungsgemeinschaft (SFB TRR219, Project-ID 322900939), and Deutsche Herzstiftung. His institution (Saarland University) has received scientific support from Ablative Solutions, Medtronic, and ReCor Medical. He has received speaker honoraria/consulting fees from Ablative Solutions, Amgen, Astra-Zeneca, Bayer, Boehringer Ingelheim, Inari Medical, Medtronic, Merck, ReCor Medical, Servier, and Terumo. Bernhard Gebauer reports consulting fees, speaking fees, and research funds to the institution from Inari Medical, BAYER, CALYX, Boston Scientific, ICON, Terumo, SIRTex Medical, and Siemens Healthineers. Asger Andersen reports speaking fees from Abbott, Gore Medical, Angiodynamics, EPS Vascular, and Jannsen and consulting fees from Inari Medical and Magneto thrombectomy solutions. Oren Friedman reports consulting fees from Inari Medical. Ripal Gandhi reports consulting fees and speaking fees from Boston Scientific, Inari Medical, Medtronic, Argon Medical, and Penumbra. Wissam Jaber reports consulting fees from Inari Medical, Medtronic, RapidAI, Abbott, and equity in Thrombolex. Keith Pereira reports having no conflicts relevant to this work. Frances West reports consulting and speaking fees from Inari Medical and Penumbra and serves on the board of directors for the PERT Consortium.
Figures
References
-
- Konstantinides S.V., Meyer G., Becattini C., et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS) Eur Heart J. 2020;41(4):543–603. - PubMed
-
- Jaff M.R., McMurtry M.S., Archer S.L., et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123(16):1788–1830. - PubMed
-
- Chatterjee S., Chakraborty A., Weinberg I., et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA. 2014;311(23):2414–2421. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

