A matching-adjusted indirect comparison of centanafadine versus lisdexamfetamine, methylphenidate and atomoxetine in adults with attention-deficit/hyperactivity disorder: long-term safety and efficacy
- PMID: 39132746
- PMCID: PMC11363209
- DOI: 10.57264/cer-2024-0089
A matching-adjusted indirect comparison of centanafadine versus lisdexamfetamine, methylphenidate and atomoxetine in adults with attention-deficit/hyperactivity disorder: long-term safety and efficacy
Abstract
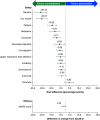
Aim: To compare long-term safety and efficacy outcomes of centanafadine versus lisdexamfetamine dimesylate (lisdexamfetamine), methylphenidate hydrochloride (methylphenidate) and atomoxetine hydrochloride (atomoxetine), respectively, in adults with attention-deficit/hyperactivity disorder (ADHD) using matching-adjusted indirect comparisons (MAICs). Patients & methods: Patient-level data from a centanafadine trial (NCT03605849) and published aggregate data from a lisdexamfetamine trial (NCT00337285), a methylphenidate trial (NCT00326300) and an atomoxetine trial (NCT00190736) were used. Patient characteristics were matched in each comparison using propensity score weighting. Study outcomes were assessed up to 52 weeks and included safety (rates of adverse events [AEs]) and efficacy (mean change from baseline in the Adult ADHD Investigator Symptom Rating Scale [AISRS] or ADHD Rating Scale [ADHD-RS] score). Results: In all comparisons of matched populations, risks of AEs were statistically significantly lower with centanafadine or non-different between centanafadine and comparator; the largest differences in AE rates included upper respiratory tract infection (risk difference in percentage points: 18.75), insomnia (12.47) and dry mouth (12.33) versus lisdexamfetamine; decreased appetite (20.25), headache (18.53) and insomnia (12.65) versus methylphenidate; and nausea (26.18), dry mouth (25.07) and fatigue (13.95) versus atomoxetine (all p < 0.05). Centanafadine had a smaller reduction in the AISRS/ADHD-RS score versus lisdexamfetamine (6.15-point difference; p < 0.05) and no statistically significant difference in the change in AISRS score versus methylphenidate (1.75-point difference; p = 0.13) and versus atomoxetine (1.60-point difference; p = 0.21). Conclusion: At up to 52 weeks, centanafadine showed significantly lower incidence of several AEs than lisdexamfetamine, methylphenidate and atomoxetine; efficacy was lower than lisdexamfetamine and non-different from methylphenidate and atomoxetine.
Keywords: adverse events; attention-deficit/hyperactivity disorder; centanafadine; clinical trials; comparative effectiveness research; efficacy; indirect comparison; propensity score; treatment outcome.
Conflict of interest statement
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Figures



References
-
- Faraone SV, Asherson P, Banaschewski T et al. Attention-deficit/hyperactivity disorder. Nat. Rev. Dis. Primers 1, 15020 (2015). - PubMed
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition (DSM-5). American Psychiatric Publishing, USA: (2013).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
