Alterations in the gut microbiome and metabolism profiles reveal the possible molecular mechanism of renal injury induced by hyperuricemia in a mouse model of renal insufficiency
- PMID: 39132829
- PMCID: PMC11321104
- DOI: 10.1080/0886022X.2024.2387429
Alterations in the gut microbiome and metabolism profiles reveal the possible molecular mechanism of renal injury induced by hyperuricemia in a mouse model of renal insufficiency
Abstract
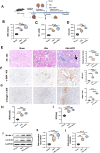
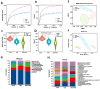
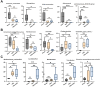
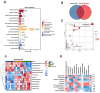
Objectives: To investigate the role of the intestinal flora and metabolites in the development of hyperuricemic renal injury in chronic kidney disease (CKD).Methods: Unilaterally nephrectomized mice were fed with adenine and potassium oxonate for 9 weeks. HE staining combined with plasma biochemical indicators was used to evaluate renal pathological and functional changes. We conducted 16S rRNA sequencing and untargeted metabolomics on feces and plasma samples to reveale changes in intestinal microbiota and metabolites.Result: Our analysis revealed significant differences in 15 bacterial genera, with 7 being upregulated and 8 being downregulated. Furthermore, metabolomic analysis revealed changes in the distribution of amino acid and biotin metabolites in basic metabolic pathways in both feces and serum. Specifically, differentially abundant metabolites in feces were associated primarily with histidine metabolism; the biosynthesis of phenylalanine, tyrosine, and tryptophan; and tyrosine metabolism. In plasma, the differentially abundant metabolites were involved in multiple metabolic pathways, including aminoacyl-tRNA biosynthesis; glycine, serine, and threonine amino acid metabolism; valine, leucine, and isoleucine biosynthesis; tyrosine biosynthesis and metabolism; biotin metabolism; and taurine and hypotaurine metabolism. Furthermore, correlation analysis revealed that Akkermansia, UCG-005, Lachnospiraceae_NK4A136_group, Lactococcus, and Butymonas were associated with various differentially abundant metabolites as well as renal function, oxidative stress, and mitophagy. The changes in the intestinal flora observed in hyperuricemia may lead to imbalances in amino acid and biotin metabolism in both the intestine and host, ultimately affecting oxidative stress and mitophagy in mice and accelerating the progression of CKD.Conclusion: Our findings provide insights into a potential pathogenic mechanism by which hyperuricemia exacerbates renal injury in mice with renal insufficiency. Understanding these pathways may offer new therapeutic strategies for managing hyperuricemic renal injury in CKD patients.
Keywords: Chronic kidney disease; gut microbiota; hyperuricemia; metabolism profiling; renal injury.
Conflict of interest statement
Upon manuscript submission, all the authors completed the author disclosure form. The authors declare that they have no competing interests.
Figures

References
-
- Choe J, Park KY, Kim SK.. Oxidative stress by monosodium urate crystals promotes renal cell apoptosis through mitochondrial caspase-dependent pathway in human embryonic kidney 293 cells: mechanism for urate-induced nephropathy. Apoptosis. 2014;20(1):38–49. doi: 10.1007/s10495-014-1057-1. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
