A promoter-RBS library for fine-tuning gene expression in Methanosarcina acetivorans
- PMID: 39132998
- PMCID: PMC11409679
- DOI: 10.1128/aem.01092-24
A promoter-RBS library for fine-tuning gene expression in Methanosarcina acetivorans
Abstract
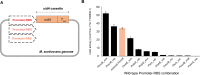
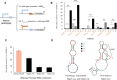
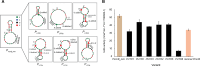
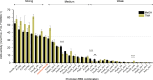
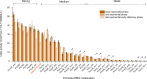
Methanogens are the main biological producers of methane on Earth. Methanosarcina acetivorans is one of the best characterized methanogens that has powerful genetic tools for genome editing. To study the physiology of this methanogen in further detail as well as to effectively balance the flux of their engineered metabolic pathways in expansive project undertakings, there is the need for controlled gene expression, which then requires the availability of well-characterized promoters and ribosome-binding sites (RBS). In this study, we constructed a library of 33 promoter-RBS combinations that includes 13 wild-type and 14 hybrid combinations, as well as six combination variants in which the 5'-untranslated region (5'UTR) was rationally engineered. The expression strength for each combination was calculated by inducing the expression of the β-glucuronidase reporter gene in M. acetivorans cells in the presence of the two most used growth substrates, either methanol (MeOH) or trimethyl amine (TMA). In this study, the constructed library covers a relatively wide range (140-fold) between the weakest and strongest promoter-RBS combination as well as shows a steady increase and allows different levels of gene expression. Effects on the gene expression strength were also assessed by making measurements at three distinct growth phases for all 33 promoter-RBS combinations. Our promoter-RBS library is effective in enabling the fine-tuning of gene expression in M. acetivorans for physiological studies and the design of metabolic engineering projects that, e.g., aim for the biotechnological valorization of one-carbon compounds.
Importance: Methanogenic archaea are potent producers of the greenhouse gas methane and thus contribute substantially to global warming. Under controlled conditions, these microbes can catalyze the production of biogas, which is a renewable fuel, and might help counter global warming and its effects. Engineering the primary metabolism of Methanosarcina acetivorans to render it better and more useful requires controllable gene expression, yet only a few well-characterized promoters and RBSs are presently available. Our study rectifies this situation by providing a library of 33 different promoter-RBS combinations with a 140-fold dynamic range in expression strength. Future metabolic engineering projects can take advantage of this library by using these promoter-RBS combinations as an efficient and tunable gene expression system for M. acetivorans. Furthermore, the methodologies we developed in this study could also be utilized to construct promoter libraries for other types of methanogens.
Keywords: 5'UTR-engineering; Methanosarcina acetivorans; RBS; gene expression; library construction; methanogens; promoter.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Deppenmeier U, Johann A, Hartsch T, Merkl R, Schmitz RA, Martinez-Arias R, Henne A, Wiezer A, Bäumer S, Jacobi C, Brüggemann H, Lienard T, Christmann A, Bömeke M, Steckel S, Bhattacharyya A, Lykidis A, Overbeek R, Klenk H-P, Gunsalus RP, Fritz H-J, Gottschalk G. 2002. The genome of Methanosarcina mazei: evidence for lateral gene transfer between bacteria and archaea. J Mol Microbiol Biotechnol 4:453–461. - PubMed
-
- Maeder DL, Anderson I, Brettin TS, Bruce DC, Gilna P, Han CS, Lapidus A, Metcalf WW, Saunders E, Tapia R, Sowers KR. 2006. The Methanosarcina barkeri genome: comparative analysis with Methanosarcina acetivorans and Methanosarcina mazei reveals extensive rearrangement within methanosarcinal genomes. J Bacteriol 188:7922–7931. doi: 10.1128/JB.00810-06 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

