Regulation of potassium uptake in Caulobacter crescentus
- PMID: 39133005
- PMCID: PMC11411941
- DOI: 10.1128/jb.00107-24
Regulation of potassium uptake in Caulobacter crescentus
Abstract
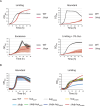
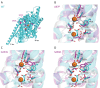
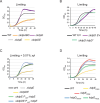
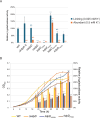
Potassium (K+) is an essential physiological element determining membrane potential, intracellular pH, osmotic/turgor pressure, and protein synthesis in cells. Here, we describe the regulation of potassium uptake systems in the oligotrophic α-proteobacterium Caulobacter crescentus known as a model for asymmetric cell division. We show that C. crescentus can grow in concentrations from the micromolar to the millimolar range by mainly using two K+ transporters to maintain potassium homeostasis, the low-affinity Kup and the high-affinity Kdp uptake systems. When K+ is not limiting, we found that the kup gene is essential while kdp inactivation does not impact the growth. In contrast, kdp becomes critical but not essential and kup dispensable for growth in K+-limited environments. However, in the absence of kdp, mutations in kup were selected to improve growth in K+-depleted conditions, likely by increasing the affinity of Kup for K+. In addition, mutations in the KdpDE two-component system, which regulates kdpABCDE expression, suggest that the inner membrane sensor regulatory component KdpD mainly works as a phosphatase to limit the growth when cells reach late exponential phase. Our data therefore suggest that KdpE is phosphorylated by another non-cognate histidine kinase. On top of this, we determined the KdpE-dependent and independent K+ transcriptome. Together, our work illustrates how an oligotrophic bacterium responds to fluctuation in K+ availability.IMPORTANCEPotassium (K+) is a key metal ion involved in many essential cellular processes. Here, we show that the oligotroph Caulobacter crescentus can support growth at micromolar concentrations of K+ by mainly using two K+ uptake systems, the low-affinity Kup and the high-affinity Kdp. Using genome-wide approaches, we also determined the entire set of genes required for C. crescentus to survive at low K+ concentration as well as the full K+-dependent regulon. Finally, we found that the transcriptional regulation mediated by the KdpDE two-component system is unconventional since unlike Escherichia coli, the inner membrane sensor regulatory component KdpD seems to work rather as a phosphatase on the phosphorylated response regulator KdpE~P.
Keywords: KdpD; KdpE; Kup; potassium transport; two-component system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

