The tandem CD33-CLL1 CAR-T as an approach to treat acute myeloid leukemia
- PMID: 39133622
- PMCID: PMC12274197
- DOI: 10.2450/BloodTransfus.786
The tandem CD33-CLL1 CAR-T as an approach to treat acute myeloid leukemia
Abstract
Background: Acute myeloid leukemia (AML) is characterized by high heterogeneity, poor long-term survival, and a propensity for relapse. Exceptional efficacy in treating recurrent or refractory B-lymphoid malignancies has been demonstrated by Chimeric antigen receptor T cells (CAR-T cells). Given the therapeutic potential of targeting both CD33 and C-type lectin-like molecule-1 (CLL1) in AML, the development of a dual-targeting CD33-CLL1 CAR-T cells assumes significant importance.
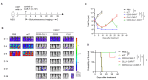
Materials and methods: The expressions of CD33 and CLL-1 antigens in peripheral blood cells and bone marrow cells from AML patients was assessed. Subsequently, a Chimeric Antigen Receptor (CAR) incorporating a dual-specific single-chain variable fragment targeting CLL1 and CD33 (CD33-CLL1-CAR-T) was engineered. The anti-tumor efficacy and potential side effects of CD33-CLL1-CAR-T cells were comprehensively investigated in both in vitro and in vivo settings.
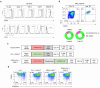
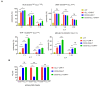
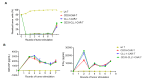
Results: The constructed tandem CD33-CLL1 CAR-T exhibited potent cytotoxicity against leukemia cell lines and human primary AML cells in vitro. Co-cultivation of AML blasts with CD33-CLL1-CAR-T cells resulted in effective proliferation and the secretion of substantial quantities of GM-CSF and IFN-γ. Importantly, the impact of CD33-CLL1-CAR-T cells on normal hematopoietic stem cells was minimal, ensuring safety in vivo mouse models. Notably, significant anti-leukemic activity was observed in the mouse model, with CD33-CLL1-CAR-T cells leading to tumor eradication and prolonged survival.
Discussion: The tandem CD33-CLL1 CAR-T cells not only efficiently eliminated AML blasts but also exhibited low cytotoxicity toward normal hematopoietic stem cells (HSCs). These findings underscore the potential clinical applicability of the tandem CD33-CLL1 CAR-T cells as an effective and safe treatment strategy for AML, representing a noteworthy advancement in the field of CAR-T cells therapy.
Conflict of interest statement
The Authors declare no conflicts of interest.
Figures

Similar articles
-
CD70 CAR T cells secreting an anti-CD33/anti-CD3 dual-targeting antibody overcome antigen heterogeneity in AML.Blood. 2025 Feb 13;145(7):720-731. doi: 10.1182/blood.2023023210. Blood. 2025. PMID: 39571145
-
JAK-STAT-activated, fratricide-resistant CAR-T cells targeting membrane-bound TNF effectively treat AML and solid tumors.J Immunother Cancer. 2025 Jul 13;13(7):e011067. doi: 10.1136/jitc-2024-011067. J Immunother Cancer. 2025. PMID: 40659447 Free PMC article.
-
CD33-D2 isoform characterization for advancement of its therapeutic potential.Immunotherapy. 2025 Apr;17(5):347-354. doi: 10.1080/1750743X.2025.2493038. Epub 2025 Apr 24. Immunotherapy. 2025. PMID: 40272002
-
CAR-T cell therapy for cancer: current challenges and future directions.Signal Transduct Target Ther. 2025 Jul 4;10(1):210. doi: 10.1038/s41392-025-02269-w. Signal Transduct Target Ther. 2025. PMID: 40610404 Free PMC article. Review.
-
Evolving strategies to overcome barriers in CAR-T cell therapy for acute myeloid leukemia.Expert Rev Hematol. 2024 Nov;17(11):797-818. doi: 10.1080/17474086.2024.2420614. Epub 2024 Oct 30. Expert Rev Hematol. 2024. PMID: 39439295 Review.
Cited by
-
Tandem CAR-T cell therapy: recent advances and current challenges.Front Immunol. 2025 Feb 28;16:1546172. doi: 10.3389/fimmu.2025.1546172. eCollection 2025. Front Immunol. 2025. PMID: 40092990 Free PMC article. Review.
-
Membrane Antigen Targeting in Acute Myeloid Leukemia Using Antibodies or CAR-T Cells.Cancers (Basel). 2024 Oct 28;16(21):3627. doi: 10.3390/cancers16213627. Cancers (Basel). 2024. PMID: 39518068 Free PMC article. Review.
-
Discovery and preclinical development of a SdAb-based CAR-T technology for targeting CD33 in AML.Mol Ther Oncol. 2025 Feb 11;33(1):200949. doi: 10.1016/j.omton.2025.200949. eCollection 2025 Mar 20. Mol Ther Oncol. 2025. PMID: 40084273 Free PMC article.
-
Targeting cancer stem cells with CAR-based immunotherapy: biology, evidence, and future directions.Cancer Cell Int. 2025 Jul 28;25(1):289. doi: 10.1186/s12935-025-03846-3. Cancer Cell Int. 2025. PMID: 40722155 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
