Resveratrol attenuates non-steroidal anti-inflammatory drug-induced intestinal injury in rats in a high-altitude hypoxic environment by modulating the TLR4/NFκB/IκB pathway and gut microbiota composition
- PMID: 39133675
- PMCID: PMC11318858
- DOI: 10.1371/journal.pone.0305233
Resveratrol attenuates non-steroidal anti-inflammatory drug-induced intestinal injury in rats in a high-altitude hypoxic environment by modulating the TLR4/NFκB/IκB pathway and gut microbiota composition
Abstract
Introduction: Non-steroidal anti-inflammatory drugs (NSAIDs) are currently the most widely used anti-inflammatory medications, but their long-term use can cause damage to the gastrointestinal tract(GIT). One of the risk factors for GIT injury is exposure to a high-altitude hypoxic environment, which can lead to damage to the intestinal mucosal barrier. Taking NSAIDs in a high-altitude hypoxic environment can exacerbate GIT injury and impact gut microbiota. The aim of this study is to investigate the mechanisms by which resveratrol (RSV) intervention alleviates NSAID-induced intestinal injury in a high-altitude hypoxic environment, as well as its role in regulating gut microbiota.
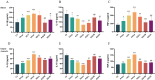
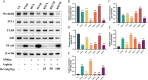
Methods: Aspirin was administered orally to rats to construct a rat model of intestinal injury induced by NSAIDs. Following the induction of intestinal injury, rats were administered RSV by gavage, and the expression levels of TLR4, NF-κB,IκB as well as Zonula Occludens-1 (ZO-1) and Occludin proteins in the different treatment groups were assessed via Western blot. Furthermore, the expression of the inflammatory factors IL-10, IL-1β, and TNF-α was evaluated using Elisa.16sRNA sequencing was employed to investigate alterations in the gut microbiota.
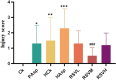
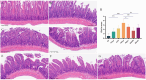
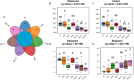
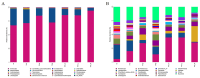
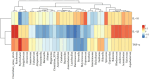
Results: The HCk group showed elevated expression of TLR4/NF-κB/IκB pathway proteins, increased expression of pro-inflammatory factors IL-1β and TNF-α, decreased expression of the anti-inflammatory factor IL-10, and expression of intestinal mucosal barrier proteins ZO-1 and Occludin. The administration of NSAIDs drugs in the plateau hypoxic environment exacerbates intestinal inflammation and damage to the intestinal mucosal barrier. After treatment with RSV intervention, the expression of TLR4/NF-κB/IκB signaling pathway proteins would be reduced, thereby lowering the expression of inflammatory factors in the HAsp group. The results of HE staining directly show the damage to the intestines and the repair of intestinal mucosa after RSV intervention. 16sRNA sequencing results show significant differences (P<0.05) in Ruminococcus, Facklamia, Parasutterella, Jeotgalicoccus, Coprococcus, and Psychrobacter between the HCk group and the Ck group. Compared to the HCk group, the HAsp group shows significant differences (P<0.05) in Facklamia, Jeotgalicoccus, Roseburia, Psychrobacter, and Alloprevotella. After RSV intervention, Clostridium_sensu_stricto bacteria significantly increase compared to the HAsp group.
Conclusion: Resveratrol can attenuate intestinal damage caused by the administration of NSAIDs at high altitude in hypoxic environments by modulating the TLR4/NF-κB/IκB signaling pathway and gut microbiota composition.
Copyright: © 2024 Xue et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
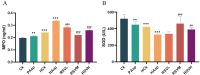

References
-
- Teutsch B.; Boros E.; Váncsa S.; Váradi A.; Frim L.; Kiss S.; et al. Mucoprotective drugs can prevent and treat nonsteroidal anti-inflammatory drug-induced small bowel enteropathy: a systematic review and meta-analysis of randomized controlled trials. Therap Adv Gastroenterol 2021, 14, 17562848211038772, doi: 10.1177/17562848211038772 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

