Post-marketing quality surveillance of selected antibacterial agents marketed in porous borders; the case of Ethiopia-Sudan-Eritrea border
- PMID: 39133679
- PMCID: PMC11318851
- DOI: 10.1371/journal.pone.0308223
Post-marketing quality surveillance of selected antibacterial agents marketed in porous borders; the case of Ethiopia-Sudan-Eritrea border
Abstract
Background: The presence of poor-quality medicines is becoming a public health threat in many parts of the world, particularly in developing countries. Antibiotics are among the most common anti-infective medicines that are highly prone to this problem.
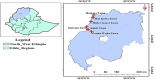
Objectives: The purpose of this study was to assess the quality of selected antibacterials that are marketed in Setit Humera and West Gondar Zones, North West Ethiopia, which are located on the Ethiopian side of the Ethiopia-Sudan-Eritrea border.
Methods: Seventy-one samples of the four antibacterial medicines (Ciprofloxacin, Norfloxacin, Amoxycillin, and Amoxycillin clavullanate combination) were collected from six sites in Setit Humera and West Gondar Zones, North West Ethiopia. A mystery shopping system was used for sample collection. Visual inspections and confirmation of the registration status were carried out using the joint WHO/FIP/USP checklist and the Ethiopian Food and Drug Authority's (EFDA's) Electronic Regulatory Information System (eRIS), respectively. Then Pharmacopeial methods (USP, BP) were employed to assess the physicochemical quality parameters.
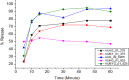
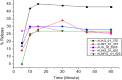
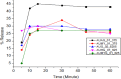
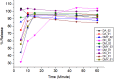
Results: During the period of our data collection, the application status for registration in the eRIS system was checked. From 71 samples, 25.35% (18/71) were not registered, and 15.49% (11/71) were registered, but the license period had expired. Some samples (12.06% (17/71)) did not meet the visual inspection criteria. The physicochemical evaluation showed that all the samples studied met the quality specifications for the identification and hardness tests. However, concerning assay, dissolution, uniformity of dosage units, disintegration, and friability test parameters, 27.49% (23/71), 16.9% (12/71), and 14.08% (10/71), 2.82% (2/71) and 8.57% (3/35) of samples were found to be substandard, respectively. Overall, 56.33% (40/71) of the samples tested were of poor quality, failing to meet any one or more of the assessed parameters in this study.
Conclusion: The study indicated that poor-quality antibacterial medicines are circulating in the study sites. Therefore, even if the area was affected by conflict at the time of the study, the regulatory bodies should focus on enforcing the necessary measures by collaborating with the regional and national regulatory medicine agencies to ensure that the antibacterial medicines available meet the required mandatory minimum standards.
Copyright: © 2024 Denekew et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Etebu E, Arikekpar I. Antibiotics: Classification and mechanisms of action with emphasis on molecular perspectives Ebimieowei. Int J Appl Microbiol Biotechnol Res. 2016;4:90–101.
-
- Jayakar B, Aleykutty NA, Mathews SM. Changes in daily defined doses (DDD) of antibiotics after restricted use in medical inpatients. J Appl Pharm Sci. 2011;1(6):220–2.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

