The hare syphilis agent is related to, but distinct from, the treponeme causing rabbit syphilis
- PMID: 39133700
- PMCID: PMC11318916
- DOI: 10.1371/journal.pone.0307196
The hare syphilis agent is related to, but distinct from, the treponeme causing rabbit syphilis
Abstract
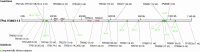
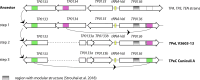
The treponemes infecting lagomorphs include Treponema paraluisleporidarum ecovar Cuniculus (TPeC) and ecovar Lepus (TPeL), infecting rabbits and hares, respectively. In this study, we described the first complete genome sequence of TPeL, isolate V3603-13, from an infected mountain hare (Lepus timidus) in Sweden. In addition, we determined 99.0% of the genome sequence of isolate V246-08 (also from an infected mountain hare, Sweden) and 31.7% of the genome sequence of isolate Z27 A77/78 (from a European hare, Lepus europeaus, The Netherlands). The TPeL V3603-13 genome had considerable gene synteny with the TPeC Cuniculi A genome and with the human pathogen T. pallidum, which causes syphilis (ssp. pallidum, TPA), yaws (ssp. pertenue, TPE) and endemic syphilis (ssp. endemicum, TEN). Compared to the TPeC Cuniculi A genome, TPeL V3603-13 contained four insertions and 11 deletions longer than three nucleotides (ranging between 6 and2,932 nts). In addition, there were 25 additional indels, from one to three nucleotides long, altogether spanning 36 nts. The number of single nucleotide variants (SNVs) between TPeC Cuniculi A and TPeL V3603-13 were represented by 309 nucleotide differences. Major proteome coding differences between TPeL and TPeC were found in the tpr gene family, and (predicted) genes coding for outer membrane proteins, suggesting that these components are essential for host adaptation in lagomorph syphilis. The phylogeny revealed that the TPeL sample from the European brown hare was more distantly related to TPeC Cuniculi A than V3603-13 and V246-08.
Copyright: © 2024 Pospíšilová et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Identification of positively selected genes in human pathogenic treponemes: Syphilis-, yaws-, and bejel-causing strains differ in sets of genes showing adaptive evolution.PLoS Negl Trop Dis. 2019 Jun 19;13(6):e0007463. doi: 10.1371/journal.pntd.0007463. eCollection 2019 Jun. PLoS Negl Trop Dis. 2019. PMID: 31216284 Free PMC article.
-
First report of hare treponematosis seroprevalence of European brown hares (Lepus europaeus) in the Czech Republic: seroprevalence negatively correlates with altitude of sampling areas.BMC Vet Res. 2019 Oct 18;15(1):350. doi: 10.1186/s12917-019-2086-3. BMC Vet Res. 2019. PMID: 31627750 Free PMC article.
-
Is there a difference between hare syphilis and rabbit syphilis? Cross infection experiments between rabbits and hares.Vet Microbiol. 2013 May 31;164(1-2):190-4. doi: 10.1016/j.vetmic.2013.02.001. Epub 2013 Feb 11. Vet Microbiol. 2013. PMID: 23473645
-
Genetics of human and animal uncultivable treponemal pathogens.Infect Genet Evol. 2018 Jul;61:92-107. doi: 10.1016/j.meegid.2018.03.015. Epub 2018 Mar 22. Infect Genet Evol. 2018. PMID: 29578082 Review.
-
Genetic diversity in Treponema pallidum: implications for pathogenesis, evolution and molecular diagnostics of syphilis and yaws.Infect Genet Evol. 2012 Mar;12(2):191-202. doi: 10.1016/j.meegid.2011.12.001. Epub 2011 Dec 15. Infect Genet Evol. 2012. PMID: 22198325 Free PMC article. Review.
References
-
- Turner TB, Hollander DH. Biology of the treponematoses based on studies carried out at the International Treponematosis Laboratory Center of the Johns Hopkins University under the auspices of the World Health Organization. Monogr Ser World Health Organ. 1957;(35):3–266. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

