Evolution of a Strategy for the Unified, Asymmetric Total Syntheses of DMOA-Derived Spiromeroterpenoids
- PMID: 39133739
- PMCID: PMC11382302
- DOI: 10.1021/acs.joc.4c01116
Evolution of a Strategy for the Unified, Asymmetric Total Syntheses of DMOA-Derived Spiromeroterpenoids
Abstract
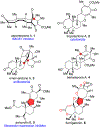
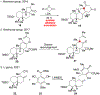
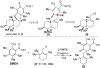
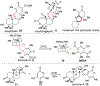
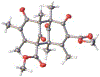
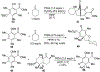
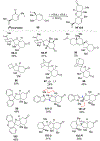
DMOA-derived spiromeroterpenoids are a group of natural products with complex structures and varied biological activities. Recently, we reported the first enantioselective total synthesis of five spiromeroterpenoids based on a fragment coupling strategy. This full account describes details of a strategy evolution that culminated in successful syntheses of the targeted natural products. Although our alkylative dearomatization methodology was unable to deliver the desired spirocyclic products in our first-generation approach, our second-generation approach based on oxidative [3 + 2] cycloaddition produced the asnovolin H core along with several complex dimers. Challenges with the dearomatization approach finally led us to develop a third generation, non-dearomatization approach based on a fragment coupling strategy to construct the conserved, sterically hindered bis-neopentyl linkage of the spiromeroterpenoids through 1,2-addition. To enable scalable access of the natural products, a refined, multigram-scale synthesis of the coupling partners was developed. A series of stereoselective transformations were developed through judicious choice of reagents and conditions. Finally, modular spirocycle construction logic was demonstrated through the synthesis of a small library of spiromeroterpenoid analogues.
Figures









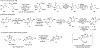


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

