Caught in a trap: DNA contamination in tsetse xenomonitoring can lead to over-estimates of Trypanosoma brucei infection
- PMID: 39133740
- PMCID: PMC11341098
- DOI: 10.1371/journal.pntd.0012095
Caught in a trap: DNA contamination in tsetse xenomonitoring can lead to over-estimates of Trypanosoma brucei infection
Abstract
Background: Tsetse flies (Glossina sp.) are vectors of Trypanosoma brucei subspecies that cause human African trypanosomiasis (HAT). Capturing and screening tsetse is critical for HAT surveillance. Classically, tsetse have been microscopically analysed to identify trypanosomes, but this is increasingly replaced with molecular xenomonitoring. Nonetheless, sensitive T. brucei-detection assays, such as TBR-PCR, are vulnerable to DNA cross-contamination. This may occur at capture, when often multiple live tsetse are retained temporarily in the cage of a trap. This study set out to determine whether infected tsetse can contaminate naïve tsetse with T. brucei DNA via faeces when co-housed.
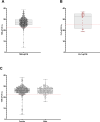
Methodology/principle findings: Insectary-reared teneral G. morsitans morsitans were fed an infectious T. b. brucei-spiked bloodmeal. At 19 days post-infection, infected and naïve tsetse were caged together in the following ratios: (T1) 9:3, (T2) 6:6 (T3) 1:11 and a control (C0) 0:12 in triplicate. Following 24-hour incubation, DNA was extracted from each fly and screened for parasite DNA presence using PCR and qPCR. All insectary-reared infected flies were positive for T. brucei DNA using TBR-qPCR. However, naïve tsetse also tested positive. Even at a ratio of 1 infected to 11 naïve flies, 91% of naïve tsetse gave positive TBR-qPCR results. Furthermore, the quantity of T. brucei DNA detected in naïve tsetse was significantly correlated with cage infection ratio. With evidence of cross-contamination, field-caught tsetse from Tanzania were then assessed using the same screening protocol. End-point TBR-PCR predicted a sample population prevalence of 24.8%. Using qPCR and Cq cut-offs optimised on insectary-reared flies, we estimated that prevalence was 0.5% (95% confidence interval [0.36, 0.73]).
Conclusions/significance: Our results show that infected tsetse can contaminate naïve flies with T. brucei DNA when co-caged, and that the level of contamination can be extensive. Whilst simple PCR may overestimate infection prevalence, quantitative PCR offers a means of eliminating false positives.
Copyright: © 2024 Saldanha et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Leak SGA. Tsetse Biology and Ecology. 2nd ed. Tsetse Biology and Ecology: Their Role in the Epidemiology and Control of Trypanosomosis. 2nd ed. CAB International; 1999. pp. 1–178.
-
- Ll Lloyd, Johnson WB. The Trypanosome Infections of Tsetse-flies in Northern Nigeria and a new Method of Estimation. Bull Entomol Res. 1924;14: 265–288. doi: 10.1017/S0007485300028352 - DOI
-
- Rogers DJ. Trypanosomiasis “risk” or “challenge”: a review. Acta Trop. 1985;42: 5–23. - PubMed
-
- Smith IM, Rennison DB. Some factors concerned in trypanosome challenge. Proceedings of the 7th Meeting of the International Scientific Committee on Trypanosomiasis Research. Brussels; 1958. pp. 63–66.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

