Insights into the interaction between UGGT, the gatekeeper of folding in the ER, and its partner, the selenoprotein SEP15
- PMID: 39133860
- PMCID: PMC11348098
- DOI: 10.1073/pnas.2315009121
Insights into the interaction between UGGT, the gatekeeper of folding in the ER, and its partner, the selenoprotein SEP15
Abstract
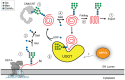
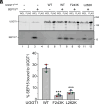
The enzyme UDP-glucose: glycoprotein glucosyltransferase (UGGT) is the gatekeeper of protein folding within the endoplasmic reticulum (ER). One-third of the human proteome traverses the ER where folding and maturation are facilitated by a complex protein homeostasis network. Both glycan modifications and disulfide bonds are of key importance in the maturation of these ER proteins. The actions of UGGT are intimately linked to the glycan code for folding and maturation of secretory proteins in the ER. UGGT selectively glucosylates the N-linked glycan of misfolded proteins so that they can reenter the lectin-folding chaperone cycle and be retained within the ER for further attempts at folding. An intriguing aspect of UGGT function is its interaction with its poorly understood cochaperone, the 15 kDa selenoprotein known as SELENOF or SEP15. This small protein contains a rare selenocysteine residue proposed to act as an oxidoreductase toward UGGT substrates. AlphaFold2 predictions of the UGGT1/SEP15 complex provide insight into this complex at a structural level. The predicted UGGT1/SEP15 interaction interface was validated by mutagenesis and coimmunoprecipitation experiments. These results serve as a springboard for models of the integrated action of UGGT1 and SEP15.
Keywords: SEP15; UGGT; endoplasmic reticulum; protein folding.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures




References
-
- Apweiler R., Hermjakob H., Sharon N., On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim. et Biophys. Acta Gen. Subj. 1473, 4–8 (1999). - PubMed
-
- Zielinska D. F., Gnad F., Wiśniewski J. R., Mann M., Precision mapping of an in vivo N-glycoproteome reveals rigid topological and sequence constraints. Cell 141, 897–907 (2010). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

