Platelet Ido1 expression is induced during Plasmodium yoelii infection, altering plasma tryptophan metabolites
- PMID: 39133890
- PMCID: PMC11609358
- DOI: 10.1182/bloodadvances.2024013175
Platelet Ido1 expression is induced during Plasmodium yoelii infection, altering plasma tryptophan metabolites
Abstract
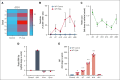
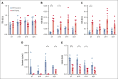
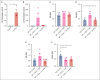
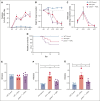
Platelets are immune responsive in many diseases as noted by changes in platelet messenger RNA in conditions such as sepsis, atherosclerosis, COVID-19, and many other inflammatory and infectious etiologies. The malaria causing Plasmodium parasite is a persistent public health threat and significant evidence shows that platelets participate in host responses to infection. Using a mouse model of nonlethal/uncomplicated malaria, non-lethal Plasmodium yoelii strain XNL (PyNL)-infected but not control mouse platelets expressed Ido1, a rate limiting enzyme in tryptophan metabolism that increases kynurenine at the expense of serotonin. Interferon-γ (IFN-γ) is a potent inducer of Ido1 and mice treated with recombinant IFN-γ had increased platelet Ido1 and IDO1 activity. PyNL-infected mice treated with anti-IFN-γ antibody had similar platelet Ido1 and metabolic profiles to that of uninfected controls. PyNL-infected mice become thrombocytopenic by day 7 after infection and transfusion of platelets from IFN-γ-treated wild-type mice but not Ido1-/- mice increased the plasma kynurenine-to-tryptophan ratio, indicating that platelets are a source of postinfection IDO1 activity. We generated platelet-specific Ido1 knockout mice to assess the contribution of platelet Ido1 during PyNL infection. Platelet-specific Ido1-/- mice had increased death and evidence of lung thrombi, which were not present in infected wild-type mice. Platelet Ido1 may be a significant contributor to plasma kynurenine in IFN-γ-driven immune processes and the loss of platelets may limit total Ido1, leading to immune and vascular dysfunction.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures


References
-
- Nurden AT, Nurden P, Sanchez M, Andia I, Anitua E. Platelets and wound healing. Front Biosci. 2008;13:3532–3548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

