A reciprocal relationship between mitochondria and lipid peroxidation determines the chondrocyte intracellular redox environment
- PMID: 39133964
- PMCID: PMC11366903
- DOI: 10.1016/j.redox.2024.103306
A reciprocal relationship between mitochondria and lipid peroxidation determines the chondrocyte intracellular redox environment
Abstract
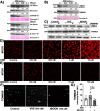
In orthopedic research, many studies have applied vitamin E as a protective antioxidant or used tert-butyl hydroperoxide to induce oxidative injury to chondrocytes. These studies often support the hypothesis that joint pathology causes oxidative stress and increased lipid peroxidation that might be prevented with lipid antioxidants to improve cell survival or function and joint health; however, lipid antioxidant supplementation was ineffective against osteoarthritis in clinical trials and animal data have been equivocal. Moreover, increased circulating vitamin E is associated with increased rates of osteoarthritis. This disconnect between benchtop and clinical results led us to hypothesize that oxidative stress-driven paradigms of chondrocyte redox function do not capture the metabolic and physiologic effects of lipid antioxidants and prooxidants on articular chondrocytes. We used ex vivo and in vivo cartilage models to investigate the effect of lipid antioxidants on healthy, primary, articular chondrocytes and applied immuno-spin trapping techniques to provide a broad indicator of high levels of oxidative stress independent of specific reactive oxygen species. Key findings demonstrate lipid antioxidants were pro-mitochondrial while lipid prooxidants decreased mitochondrial measures. In the absence of injury, radical formation was increased by lipid antioxidants; however, in the presence of injury, radical formation was decreased. In unstressed conditions, this relationship between chondrocyte mitochondria and redox regulation was reproduced in vivo with overexpression of glutathione peroxidase 4. In mice aged 18 months or more, overexpression of glutathione peroxidase 4 significantly decreased the presence of pro-mitochondrial peroxisome proliferation activated receptor gamma and deranged the relationship between mitochondria and the redox environment. This complex interaction suggests strategies targeting articular cartilage may benefit from adopting more nuanced paradigms of articular chondrocyte redox metabolism.
Keywords: Cartilage; Chondrocyte; Glutathione peroxidase 4; Immuno-spin trapping; Lipid peroxidation; Mitochondria.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest None.
Figures





References
-
- Gomez-Contreras P.C., et al. Intersections between mitochondrial metabolism and redox biology mediate posttraumatic osteoarthritis. Curr. Rheumatol. Rep. 2021;23(5):32. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

