Final Analysis Results from the AGEHA Study: Emicizumab Prophylaxis for Acquired Hemophilia A with or without Immunosuppressive Therapy
- PMID: 39134043
- PMCID: PMC12040431
- DOI: 10.1055/a-2384-3585
Final Analysis Results from the AGEHA Study: Emicizumab Prophylaxis for Acquired Hemophilia A with or without Immunosuppressive Therapy
Abstract
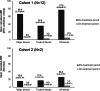
Primary analysis of the phase III AGEHA study suggested a favorable benefit-risk profile for emicizumab prophylaxis in patients with acquired hemophilia A (PwAHA); however, only patients undergoing immunosuppressive therapy (IST; Cohort 1) were included.To present final analysis results of AGEHA, including data on IST-ineligible patients (Cohort 2) and on long-term prophylaxis with emicizumab.For patients in both Cohorts 1 and 2, emicizumab was administered subcutaneously at 6 mg/kg on Day 1, 3 mg/kg on Day 2, and 1.5 mg/kg once weekly from Day 8 onward.Twelve patients (Cohort 1) and two patients (Cohort 2) were enrolled. Duration of emicizumab treatment was 8 to 639 days (median: 44.5 days) in Cohort 1 and 64 and 450 days in Cohort 2. In both cohorts, no major bleeds were observed after initial emicizumab administration. Six patients started their first rehabilitation sessions during emicizumab treatment and no rehabilitation-related bleeds occurred. Twenty-three surgeries were performed under emicizumab prophylaxis and there were no bleeds related to surgeries. Although asymptomatic deep vein thrombosis was reported in one patient in the primary analysis, no other thrombotic events occurred thereafter. Two patients developed anti-emicizumab antibodies, one of whom showed accelerated emicizumab clearance. Tailored IST approaches (delayed initiation, no use, or reduced dose) were successfully executed in three patients undergoing emicizumab prophylaxis.These results suggest that emicizumab prophylaxis has a favorable benefit-risk profile in PwAHA regardless of eligibility for IST.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Conflict of interest statement
M. Shima has received research funding from Chugai Pharmaceutical Co., Ltd., CSL Behring, and Takeda; has received honoraria from Bayer, Chugai Pharmaceutical Co., Ltd., CSL Behring, Fujimoto Seiyaku, Sanofi, Novo Nordisk, Pfizer, and Takeda; holds patents with Chugai Pharmaceutical Co., Ltd.; and has participated on a data safety monitoring board or advisory board for Chugai Pharmaceutical Co., Ltd., Fujimoto Seiyaku, KYORIN Pharmaceutical Co., Ltd., Novo Nordisk, and Pfizer. N.S. has received honoraria from Bayer, Chugai Pharmaceutical Co., Ltd., CSL Behring, Japan Blood Products Organization, KM Biologics, Novo Nordisk, Pfizer, Sanofi, and Takeda. K.A. has received research funding from KM Biologics; has received consulting fees from Chugai Pharmaceutical Co., Ltd.; has received honoraria from Bayer, Chugai Pharmaceutical Co., Ltd., CSL Behring, Fujimoto Pharmaceutical Corporation, Japan Blood Products Organization, KM Biologics, Novo Nordisk, Pfizer, Sanofi, and Takeda; has participated on a data safety monitoring board or advisory board for Chugai Pharmaceutical Co., Ltd.; and is belong to endowed chair for CSL Behring. Y.O. has received consulting fees and honoraria from Chugai Pharmaceutical Co., Ltd. R.K. and R.O. are employees of Chugai Pharmaceutical Co., Ltd. K.Y. is an employee of Chugai Pharmaceutical Co., Ltd. and an inventor of patents related to anti-activated FIX/FX bispecific antibodies. N.M. is an employee of Chugai Pharmaceutical Co., Ltd. and has stocks of Chugai Pharmaceutical Co., Ltd. E.S. has received research funding from Eisai; has received honoraria from Janssen, Novartis, Pfizer, Sanofi, and Takeda, and has leadership or fiduciary role in other board, society, committee, or advocacy group (unpaid) for Japan Adult Leukemia Study Group and Japanese Society of Myeloma. S.H. has received research funding from Chugai Pharmaceutical Co., Ltd. and has received honoraria from Bayer, Chugai Pharmaceutical Co., Ltd., CSL Behring, Novo Nordisk, Sanofi, and Takeda. K.N. has received research funding from Bayer, Chugai Pharmaceutical Co., Ltd., CSL Behring, KM Biologics, Novo Nordisk, Sanofi, and Takeda; has received consulting fees from Chugai Pharmaceutical Co., Ltd.; and has received honoraria from Bayer, Chugai Pharmaceutical Co., Ltd., CSL Behring, KM Biologics, Novo Nordisk, Sanofi, and Takeda. The remaining authors have no conflicts of interest to declare.
Figures
References
-
- UK Haemophilia Centre Doctors' Organisation . Collins P W, Hirsch S, Baglin T P et al. Acquired hemophilia A in the United Kingdom: a 2-year national surveillance study by the United Kingdom Haemophilia Centre Doctors' Organisation. Blood. 2007;109(05):1870–1877. - PubMed
-
- EACH2 Registry Contributors . Knoebl P, Marco P, Baudo F et al. Demographic and clinical data in acquired hemophilia A: results from the European Acquired Haemophilia Registry (EACH2) J Thromb Haemost. 2012;10(04):622–631. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical