Extracellular adenosine oppositely regulates the purinome machinery in glioblastoma and mesenchymal stem cells
- PMID: 39134088
- PMCID: PMC11580377
- DOI: 10.1002/iub.2905
Extracellular adenosine oppositely regulates the purinome machinery in glioblastoma and mesenchymal stem cells
Abstract
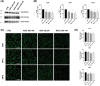
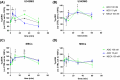
Glioblastoma (GB) is a lethal brain tumor that rapidly adapts to the dynamic changes of the tumor microenvironment (TME). Mesenchymal stem/stromal cells (MSCs) are one of the stromal components of the TME playing multiple roles in tumor progression. GB progression is prompted by the immunosuppressive microenvironment characterized by high concentrations of the nucleoside adenosine (ADO). ADO acts as a signaling molecule through adenosine receptors (ARs) but also as a genetic and metabolic regulator. Herein, the effects of high extracellular ADO concentrations were investigated in a human glioblastoma cellular model (U343MG) and MSCs. The modulation of the purinome machinery, i.e., the ADO production (CD39, CD73, and adenosine kinase [ADK]), transport (equilibrative nucleoside transporters 1 (ENT1) and 2 (ENT2)), and degradation (adenosine deaminase [ADA]) were investigated in both cell lines to evaluate if ADO could affect its cell management in a positive or negative feed-back loop. Results evidenced a different behavior of GB and MSC cells upon exposure to high extracellular ADO levels: U343MG were less sensitive to the ADO concentration and only a slight increase in ADK and ENT1 was evidenced. Conversely, in MSCs, the high extracellular ADO levels reduced the ADK, ENT1, and ENT2 expression, which further sustained the increase of extracellular ADO. Of note, MSCs primed with the GB-conditioned medium or co-cultured with U343MG cells were not affected by the increase of extracellular ADO. These results evidenced how long exposure to ADO could produce different effects on cancer cells with respect to MSCs, revealing a negative feedback loop that can support the GB immunosuppressive microenvironment. These results improve the knowledge of the ADO role in the maintenance of TME, which should be considered in the development of therapeutic strategies targeting adenosine pathways as well as cell-based strategies using MSCs.
Keywords: adenosine; glioblastoma; mesenchymal stem cells (MSC); purine metabolism; tumor microenvironment (TME).
© 2024 The Author(s). IUBMB Life published by Wiley Periodicals LLC on behalf of International Union of Biochemistry and Molecular Biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

