Encoding of 2D Self-Centered Plans and World-Centered Positions in the Rat Frontal Orienting Field
- PMID: 39134418
- PMCID: PMC11391499
- DOI: 10.1523/JNEUROSCI.0018-24.2024
Encoding of 2D Self-Centered Plans and World-Centered Positions in the Rat Frontal Orienting Field
Abstract
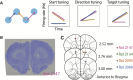
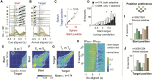
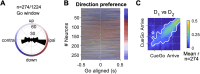
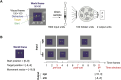
The neural mechanisms of motor planning have been extensively studied in rodents. Preparatory activity in the frontal cortex predicts upcoming choice, but limitations of typical tasks have made it challenging to determine whether the spatial information is in a self-centered direction reference frame or a world-centered position reference frame. Here, we trained male rats to make delayed visually guided orienting movements to six different directions, with four different target positions for each direction, which allowed us to disentangle direction versus position tuning in neural activity. We recorded single unit activity from the rat frontal orienting field (FOF) in the secondary motor cortex, a region involved in planning orienting movements. Population analyses revealed that the FOF encodes two separate 2D maps of space. First, a 2D map of the planned and ongoing movement in a self-centered direction reference frame. Second, a 2D map of the animal's current position on the port wall in a world-centered reference frame. Thus, preparatory activity in the FOF represents self-centered upcoming movement directions, but FOF neurons multiplex both self- and world-reference frame variables at the level of single neurons. Neural network model comparison supports the view that despite the presence of world-centered representations, the FOF receives the target information as self-centered input and generates self-centered planning signals.
Keywords: frontal cortex; motor planning; neural networks; neurophysiology; reference frame; rodent.
Copyright © 2024 Li et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures






References
MeSH terms
LinkOut - more resources
Full Text Sources
