Amyloid-β Causes NMDA Receptor Dysfunction and Dendritic Spine Loss through mGluR1 and AKAP150-Anchored Calcineurin Signaling
- PMID: 39134419
- PMCID: PMC11391497
- DOI: 10.1523/JNEUROSCI.0675-24.2024
Amyloid-β Causes NMDA Receptor Dysfunction and Dendritic Spine Loss through mGluR1 and AKAP150-Anchored Calcineurin Signaling
Abstract
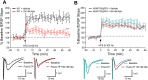
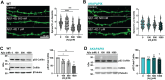
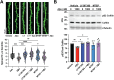
Neuronal excitatory synapses are primarily located on small dendritic protrusions called spines. During synaptic plasticity underlying learning and memory, Ca2+ influx through postsynaptic NMDA-type glutamate receptors (NMDARs) initiates signaling pathways that coordinate changes in dendritic spine structure and synaptic function. During long-term potentiation (LTP), high levels of NMDAR Ca2+ influx promote increases in both synaptic strength and dendritic spine size through activation of Ca2+-dependent protein kinases. In contrast, during long-term depression (LTD), low levels of NMDAR Ca2+ influx promote decreased synaptic strength and spine shrinkage and elimination through activation of the Ca2+-dependent protein phosphatase calcineurin (CaN), which is anchored at synapses via the scaffold protein A-kinase anchoring protein (AKAP)150. In Alzheimer's disease (AD), the pathological agent amyloid-β (Aβ) may impair learning and memory through biasing NMDAR Ca2+ signaling pathways toward LTD and spine elimination. By employing AKAP150 knock-in mice of both sexes with a mutation that disrupts CaN anchoring to AKAP150, we revealed that local, postsynaptic AKAP-CaN-LTD signaling was required for Aβ-mediated impairment of NMDAR synaptic Ca2+ influx, inhibition of LTP, and dendritic spine loss. Additionally, we found that Aβ acutely engages AKAP-CaN signaling through activation of G-protein-coupled metabotropic glutamate receptor 1 (mGluR1) leading to dephosphorylation of NMDAR GluN2B subunits, which decreases Ca2+ influx to favor LTD over LTP, and cofilin, which promotes F-actin severing to destabilize dendritic spines. These findings reveal a novel interplay between NMDAR and mGluR1 signaling that converges on AKAP-anchored CaN to coordinate dephosphorylation of postsynaptic substrates linked to multiple aspects of Aβ-mediated synaptic dysfunction.
Keywords: AKAP; NMDA receptor; amyloid-β; calcineurin; dendritic spine; mGluR1.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
