Women's health in focus: Real-world data on valproate prescriptions during pregnancy - a cohort study in Catalonia (Spain)
- PMID: 39134441
- PMCID: PMC11337672
- DOI: 10.1136/bmjopen-2024-085167
Women's health in focus: Real-world data on valproate prescriptions during pregnancy - a cohort study in Catalonia (Spain)
Abstract
Objectives: To characterise the exposure to valproate within a cohort of pregnant women using electronic health records (EHRs) from Catalonia (System for the Development of Research in Primary Care, SIDIAP).
Design: Drug-utilisation cohort study covering the period from January 2011 to June 2020. The study included pregnancy episodes of women from Catalonia identified by the algorithm.
Setting: Data were sourced from SIDIAP, a comprehensive EHR repository that includes information from various data sources: recorded prescriptions (both hospital and primary care), diagnoses and sociodemographic characteristics identified by primary care physicians, and sexual and reproductive health data from ASSIR (used by gynaecologists and midwives).
Participants: Women aged 12-50 with at least one pregnancy episode occurred during January 2011-June 2020 and at least a prescription of valproate during pregnancy.
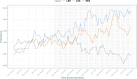
Primary and secondary outcomes: Primary outcomes included valproate exposure, measured through prevalence and cumulative incidence in pregnancy episodes and by trimester. The impact of regulatory measures (risk mitigation measures, RMMs) was assessed, and prescriptions over time were analysed using interrupted time series analysis. Secondary outcomes included health issues, pregnancy outcomes, smoking habits and socioeconomic characteristics.
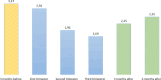
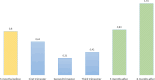
Results: A total of 99 605 pregnancies were identified, with at least 3.03‰ (95% CI 2.69‰ to 3.39‰) exposed to valproate at some point (302 pregnancies, 276 women). The median pregnancy duration was 38.30 weeks (IQR 12.6-40.1), and the median age at pregnancy was 32.37 years (IQR 27.20-36.56). Epilepsy was the most frequent health issue. The prevalence and cumulative incidence of valproate prescriptions decreased during pregnancy and increased postpregnancy. The RMMs implemented in 2014 led to a reduction in monthly valproate prescriptions during pregnancy in this cohort.
Conclusions: The study highlights the decline in valproate prescriptions during pregnancy due to RMMs and underscores the need for standardised methodologies in future studies to ensure the safety of pregnant patients and optimise scientific evidence.
Keywords: clinical pharmacology; electronic health records; maternal medicine; pregnant women; public health.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- AEMPS Spanish Agency of Medicines and Medical Devices. CIMA (Medicines Information Center). Technical data of valproic acid. Spain: Spanish Agency of Medicines and Medical Devices. 2023. https://cima.aemps.es/cima/publico/home.html Available.
-
- Spanish Agency of Medicines and Medical Devices (February 2018) Informative note: Valproic Acid: New measures to avoid exposure during pregnancy. 2018. pp. 1–3.
-
- AEMPS Spanish agency of medicines and medical devices. Pregnancy prevention program. 2018. https://www.aemps.gob.es/informa/notasinformativas/medicamentosusohumano... Available.
-
- Schachter SC, Garcia P, Dashe JF. Antiseizure medications: mechanism of action, pharmacology, and adverse effects. Waltham, MA: UpToDate; 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical