Distinctive duodenal microbiomes and bile acid profiles in duodenal tumor patients revealed by prospective observational study
- PMID: 39134638
- PMCID: PMC11319767
- DOI: 10.1038/s41598-024-69820-7
Distinctive duodenal microbiomes and bile acid profiles in duodenal tumor patients revealed by prospective observational study
Abstract
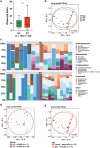
The incidence of duodenal tumors (DTs) is increasing. However, the mechanisms underlying its development remain unclear. Environmental factors, including the microbiome and bile acids (BAs), are believed to influence tumor development. Therefore, we conducted a single-center, prospective, observational study to investigate the potential differences between patients with DTs and healthy controls (HCs) based on these factors. In addition, the BAs in the duodenal fluid were measured using liquid chromatography-tandem mass spectrometry. We recruited 41 patients and performed 16S rRNA-seq. There was no difference in the observed ASVs or PCoA plot of Bray-Curtis dissimilarity between the DTs and HCs. The lithocholic acid concentration was significantly lower in the DT group than in the control group. The ratio of CDCA to LCA was significantly higher in patients with DTs. No significant differences in microbiota were observed between DTs and HCs. In patients with DTs, the lithocholic acid concentration in duodenal was significantly lower than in HCs.
© 2024. The Author(s).
Conflict of interest statement
Kentaro Miyamoto is an employee of Miyarisan Pharm. Yoko Kubosawa, Tomohisa Sujino, Atsuto Kayashima, Daisuke Minezaki, Kohei Morioka, Kentaro Iwata, Kurato Miyazaki, Teppei Masunaga, Mari Mizutani, Teppei Akimoto, Yusaku Takatori, Noriko Matsuura, Atsushi Nakayama, Kaoru Takabayashi, Nobuhiro Nakamoto, Akira Honda, Motohiko Kato, Naohisa Yahagi, Takanori Kanai have no competing interests.
Figures




References
-
- Qubaiah, O., Devesa, S. S., Platz, C. E., Huycke, M. M. & Dores, G. M. Small intestinal cancer: A population-based study of incidence and survival patterns in the United States, 1992 to 2006. Cancer Epidemiol. Biomarkers Prev.19, 1908–1918. 10.1158/1055-9965.Epi-10-0328 (2010). 10.1158/1055-9965.Epi-10-0328 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

