Multiple Severe Intracranial Stenoses with Ischemic Stroke in Neuroborreliosis-associated Cerebral Vasculitis: Endovascular Treatment Strategies and Literature Review
- PMID: 39134673
- PMCID: PMC11564264
- DOI: 10.1007/s00062-024-01447-7
Multiple Severe Intracranial Stenoses with Ischemic Stroke in Neuroborreliosis-associated Cerebral Vasculitis: Endovascular Treatment Strategies and Literature Review
Abstract
Introduction: Neuroborreliosis is the disseminated form of Lyme borreliosis and refers to the involvement of the central nervous system by Borrelia burgdorferi sensu lato spirochetes. Several reports suggest its emergence as a potential cause of cerebral vasculitis and stroke in children and young adults. The objective of this paper is to highlight endovascular treatment options within this context.
Methods: The medicinal and endovascular treatments of three patients-two adults and one child-with ischemic stroke resulting from neuroborreliosis-associated severe cerebral vasculitis were retrospectively assessed. Detailed descriptions of the clinical course, treatments, and follow-up data for each patient are provided. Additionally, a literature review focusing on endovascular treatment options within this topic was conducted.
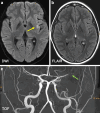
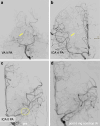
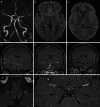
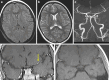
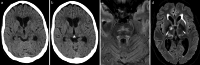
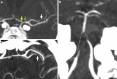
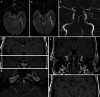
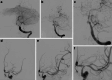
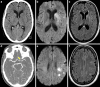
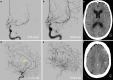
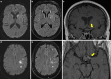
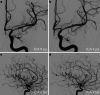
Results: Both endovascular and medicinal treatments resulted in excellent clinical outcomes in all three patients, with no observed periprocedural complications. Significant clinical improvement was noted during mid-term follow-up. Follow-up angiographies confirmed stent patency.
Conclusion: Endovascular interventions as a bailout strategy may enhance clinical outcomes in patients with vascular complications of neuroborreliosis, especially when medicinal therapy alone fails to achieve further improvement. In the setting of severe ischemic stroke with sub-occlusive large vessel stenosis or occlusion, the cause of which is often unknown, it should be considered to prioritize prompt endovascular treatment, even if neuroborreliosis is suspected on admission.
Keywords: Cerebral vasculitis; Endovascular treatment; Intracranial stenting; Ischemic stroke; Lyme neuroborreliosis; Vessel wall imaging.
© 2024. The Author(s).
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

