Structural basis of LRPPRC-SLIRP-dependent translation by the mitoribosome
- PMID: 39134711
- PMCID: PMC11637978
- DOI: 10.1038/s41594-024-01365-9
Structural basis of LRPPRC-SLIRP-dependent translation by the mitoribosome
Erratum in
-
Publisher Correction: Structural basis of LRPPRC-SLIRP-dependent translation by the mitoribosome.Nat Struct Mol Biol. 2024 Nov;31(11):1809. doi: 10.1038/s41594-024-01402-7. Nat Struct Mol Biol. 2024. PMID: 39300173 Free PMC article. No abstract available.
Abstract
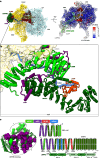
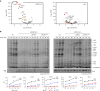
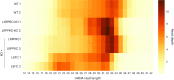
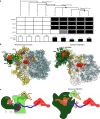
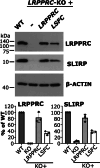
In mammalian mitochondria, mRNAs are cotranscriptionally stabilized by the protein factor LRPPRC (leucine-rich pentatricopeptide repeat-containing protein). Here, we characterize LRPPRC as an mRNA delivery factor and report its cryo-electron microscopy structure in complex with SLIRP (SRA stem-loop-interacting RNA-binding protein), mRNA and the mitoribosome. The structure shows that LRPPRC associates with the mitoribosomal proteins mS39 and the N terminus of mS31 through recognition of the LRPPRC helical repeats. Together, the proteins form a corridor for handoff of the mRNA. The mRNA is directly bound to SLIRP, which also has a stabilizing function for LRPPRC. To delineate the effect of LRPPRC on individual mitochondrial transcripts, we used RNA sequencing, metabolic labeling and mitoribosome profiling, which showed a transcript-specific influence on mRNA translation efficiency, with cytochrome c oxidase subunit 1 and 2 translation being the most affected. Our data suggest that LRPPRC-SLIRP acts in recruitment of mitochondrial mRNAs to modulate their translation. Collectively, the data define LRPPRC-SLIRP as a regulator of the mitochondrial gene expression system.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






References
-
- Sissler, M. & Hashem, Y. Mitoribosome assembly comes into view. Nat. Struct. Mol. Biol.28, 631–633 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

