Crosslinked-hybrid nanoparticle embedded in thermogel for sustained co-delivery to inner ear
- PMID: 39135039
- PMCID: PMC11321169
- DOI: 10.1186/s12951-024-02686-z
Crosslinked-hybrid nanoparticle embedded in thermogel for sustained co-delivery to inner ear
Abstract
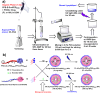
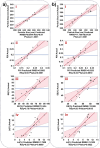
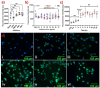
Treatment-induced ototoxicity and accompanying hearing loss are a great concern associated with chemotherapeutic or antibiotic drug regimens. Thus, prophylactic cure or early treatment is desirable by local delivery to the inner ear. In this study, we examined a novel way of intratympanically delivered sustained nanoformulation by using crosslinked hybrid nanoparticle (cHy-NPs) in a thermoresponsive hydrogel i.e. thermogel that can potentially provide a safe and effective treatment towards the treatment-induced or drug-induced ototoxicity. The prophylactic treatment of the ototoxicity can be achieved by using two therapeutic molecules, Flunarizine (FL: T-type calcium channel blocker) and Honokiol (HK: antioxidant) co-encapsulated in the same delivery system. Here we investigated, FL and HK as cytoprotective molecules against cisplatin-induced toxic effects in the House Ear Institute - Organ of Corti 1 (HEI-OC1) cells and in vivo assessments on the neuromast hair cell protection in the zebrafish lateral line. We observed that cytotoxic protective effect can be enhanced by using FL and HK in combination and developing a robust drug delivery formulation. Therefore, FL-and HK-loaded crosslinked hybrid nanoparticles (FL-cHy-NPs and HK-cHy-NPs) were synthesized using a quality-by-design approach (QbD) in which design of experiment-central composite design (DoE-CCD) following the standard least-square model was used for nanoformulation optimization. The physicochemical characterization of FL and HK loaded-NPs suggested the successful synthesis of spherical NPs with polydispersity index < 0.3, drugs encapsulation (> 75%), drugs loading (~ 10%), stability (> 2 months) in the neutral solution, and appropriate cryoprotectant selection. We assessed caspase 3/7 apopototic pathway in vitro that showed significantly reduced signals of caspase 3/7 activation after the FL-cHy-NPs and HK-cHy-NPs (alone or in combination) compared to the CisPt. The final formulation i.e. crosslinked-hybrid-nanoparticle-embedded-in-thermogel was developed by incorporating drug-loaded cHy-NPs in poloxamer-407, poloxamer-188, and carbomer-940-based hydrogel. A combination of artificial intelligence (AI)-based qualitative and quantitative image analysis determined the particle size and distribution throughout the visible segment. The developed formulation was able to release the FL and HK for at least a month. Overall, a highly stable nanoformulation was successfully developed for combating treatment-induced or drug-induced ototoxicity via local administration to the inner ear.
Keywords: Artificial intelligence image analysis; Central composite design; Deep learning model; Drug-induced-ototoxicty; Hearing loss; Local drug delivery; Long-term drug delivery; Otoprotectants; Quality-by-design approach; Redox homeostasis; Zebrafish model.
© 2024. The Author(s).
Conflict of interest statement
NST and VA declare the following competing financial interest(s): A US patent has been applied for the majority of this research work under application no. 63/509,823.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

