Neutrophil membrane-derived nanoparticles protect traumatic brain injury via inhibiting calcium overload and scavenging ROS
- PMID: 39135044
- PMCID: PMC11320991
- DOI: 10.1186/s12951-024-02753-5
Neutrophil membrane-derived nanoparticles protect traumatic brain injury via inhibiting calcium overload and scavenging ROS
Abstract
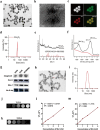
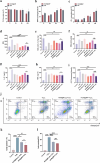
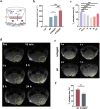
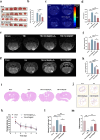
The secondary injury is more serious after traumatic brain injury (TBI) compared with primary injury. Release of excessive reactive oxygen species (ROS) and Ca2+ influx at the damaged site trigger the secondary injury. Herein, a neutrophil-like cell membrane-functionalized nanoparticle was developed to prevent ROS-associated secondary injury. NCM@MP was composed of three parts: (1) Differentiated neutrophil-like cell membrane (NCM) was synthesized, with inflammation-responsive ability to achieve effective targeting and to increase the retention time of Mn3O4 and nimodipine (MP) in deep injury brain tissue via C-X-C chemokine receptor type 4, integrin beta 1 and macrophage antigen-1. (2) Nimodipine was used to inhibit Ca2+ influx, eliminating the ROS at source. (3) Mn3O4 further eradicated the existing ROS. In addition, NCM@MP also exhibited desirable properties for T1 enhanced imaging and low toxicity which may serve as promising multifunctional nanoplatforms for precise therapies. In our study, NCM@MP obviously alleviated oxidative stress response, reduced neuroinflammation, protected blood-brain barrier integrity, relieved brain edema, promoted the regeneration of neurons, and improved the cognition of TBI mice. This study provides a promising TBI management to relieve the secondary spread of damage.
Keywords: Mn3O4; Neutrophil membrane; Nimodipine; Reactive oxygen species; Traumatic brain injury.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Kalra S, Malik R, Singh G, Bhatia S, Al-Harrasi A, Mohan S, Albratty M, Albarrati A, Tambuwala MM. Pathogenesis and management of traumatic brain injury (TBI): role of neuroinflammation and anti-inflammatory drugs. Inflammopharmacology. 2022;30:1153–66. 10.1007/s10787-022-01017-8 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 2023GDRC002/Chongqing Science and Health Joint Medical Research Project-Young and Middle-aged High-level Talent Project
- 2022XJS29/Science and Technology Innovation Ability Enhancement Project of Army Medical University
- cstc2019jcyj-msxmX0123/Natural Science Foundation of Chongqing
- CSTC2015YFPT-gcjsyjzx0175/Chongqing Clinical Research Centre of Imaging and Nuclear Medicine
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

