The role of macrophage plasticity in neurodegenerative diseases
- PMID: 39135084
- PMCID: PMC11321226
- DOI: 10.1186/s40364-024-00624-7
The role of macrophage plasticity in neurodegenerative diseases
Abstract
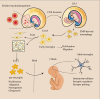
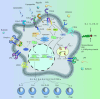
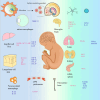
Tissue-resident macrophages and recruited macrophages play pivotal roles in innate immunity and the maintenance of brain homeostasis. Investigating the involvement of these macrophage populations in eliciting pathological changes associated with neurodegenerative diseases has been a focal point of research. Dysregulated states of macrophages can compromise clearance mechanisms for pathological proteins such as amyloid-β (Aβ) in Alzheimer's disease (AD) and TDP-43 in Amyotrophic lateral sclerosis (ALS). Additionally, recent evidence suggests that abnormalities in the peripheral clearance of pathological proteins are implicated in the pathogenesis and progression of neurodegenerative diseases. Furthermore, numerous genome-wide association studies have linked genetic risk factors, which alter the functionality of various immune cells, to the accumulation of pathological proteins. This review aims to unravel the intricacies of macrophage biology in both homeostatic conditions and neurodegenerative disorders. To this end, we initially provide an overview of the modifications in receptor and gene expression observed in diverse macrophage subsets throughout development. Subsequently, we outlined the roles of resident macrophages and recruited macrophages in neurodegenerative diseases and the progress of targeted therapy. Finally, we describe the latest advances in macrophage imaging methods and measurement of inflammation, which may provide information and related treatment strategies that hold promise for informing the design of future investigations and therapeutic interventions.
Keywords: Gene expression; Imaging methodologies; Immune cells; Macrophages; Neurodegenerative disease.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Utz SG, See P, Mildenberger W, Thion MS, Silvin A, Lutz M et al. Early fate defines microglia and non-parenchymal brain macrophage development. Cell. 2020;181:557 – 73.e18. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

