Romiplostim in chemotherapy-induced thrombocytopenia: A review of the literature
- PMID: 39135303
- PMCID: PMC11319220
- DOI: 10.1002/cam4.7429
Romiplostim in chemotherapy-induced thrombocytopenia: A review of the literature
Abstract
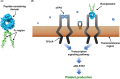
Chemotherapy-induced thrombocytopenia (CIT) is a common challenge of cancer therapy and can lead to chemotherapy dose reduction, delay, and/or discontinuation, affecting relative dose intensity, and possibly adversely impacting cancer care. Besides changing anticancer regimens, standard management of CIT has been limited to platelet transfusions and supportive care. Use of the thrombopoietin receptor agonist romiplostim, already approved for use in immune thrombocytopenia, has shown promising signs of efficacy in CIT. In a phase 2 prospective randomized study of solid tumor patients with platelet counts <100 × 109/L for ≥4 weeks due to CIT, weekly romiplostim corrected the platelet count to >100 × 109/L in 93% (14/15) of patients within 3 weeks versus 12.5% (1/8) of untreated patients (p < 0.001). Including patients treated with romiplostim in an additional single-arm cohort, 85% (44/52) of all romiplostim-treated patients responded with platelet count correction within 3 weeks. Several retrospective studies of CIT have also shown responses to weekly romiplostim, with the largest study finding that poor response to romiplostim was predicted by tumor invasion of the bone marrow (odds ratio, 0.029; 95% CI: 0.0046-0.18; p < 0.001), prior pelvic irradiation (odds ratio, 0.078; 95% CI: 0.0062-0.98; p = 0.048), and prior temozolomide treatment (odds ratio 0.24; 95% CI: 0.061-0.96; p = 0.043). Elsewhere, lower baseline TPO levels were predictive of romiplostim response (p = 0.036). No new safety signals have emerged from romiplostim CIT studies. Recent treatment guidelines, including those from the National Comprehensive Cancer Network, now support consideration of romiplostim use in CIT. Data are expected from two ongoing phase 3 romiplostim CIT trials.
Keywords: chemotherapy; peptibody; platelet growth factors; romiplostim; thrombocytopenia; thrombopoietin receptor agonists.
© 2024 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
G.A.S.: Consultant for Amgen, Janssen, Bayer Pharmaceuticals, Sobi, Bristol‐Myers Squibb, Pfizer, Novartis, Anthos Therapeutics; research funding from Amgen, Janssen, Sobi. H.A‐S consultant for Agios, Alnylam, Alpine, Amgen, Sobi, Argenx, Novartis, Pharmacosmos; research funding from Agios, Sobi, Amgen, Novartis, Vaderis. A.L.: consultation/advisory board fees from Bayer, Novartis, Pfizer, Sanofi. M.E., H.S.: Amgen employees and stockholders.
Figures

References
-
- Kuter DJ. Managing thrombocytopenia associated with cancer chemotherapy. Oncology (Williston Park). 2015;29(4):282‐294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

