Bacterial Iron Siderophore Drives Tumor Survival and Ferroptosis Resistance in a Biofilm-Tumor Spheroid Coculture Model
- PMID: 39135304
- PMCID: PMC11496991
- DOI: 10.1002/advs.202404467
Bacterial Iron Siderophore Drives Tumor Survival and Ferroptosis Resistance in a Biofilm-Tumor Spheroid Coculture Model
Abstract
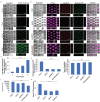
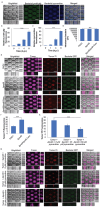
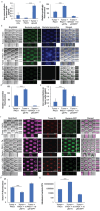
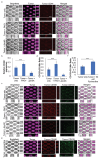
Interactions between tumoral cells and tumor-associated bacteria within the tumor microenvironment play a significant role in tumor survival and progression, potentially impacting cancer treatment outcomes. In lung cancer patients, the Gram-negative pathogen Pseudomonas aeruginosa raises questions about its role in tumor survival. Here, a microfluidic-based 3D-human lung tumor spheroid-P. aeruginosa model is developed to study the bacteria's impact on tumor survival. P. aeruginosa forms a tumor-associated biofilm by producing Psl exopolysaccharide and secreting iron-scavenging pyoverdine, which is critical for establishing a bacterial community in tumors. Consequently, pyoverdine promotes cancer progression by reducing susceptibility to iron-induced death (ferroptosis), enhancing cell viability, and facilitating several cancer hallmarks, including epithelial-mesenchymal transition and metastasis. A promising combinatorial therapy approach using antimicrobial tobramycin, ferroptosis-inducing thiostrepton, and anti-cancer doxorubicin could eradicate biofilms and tumors. This work unveils a novel phenomenon of cross-kingdom cooperation, where bacteria protect tumors from death, and it paves the way for future research in developing antibiofilm cancer therapies. Understanding these interactions offers potential new strategies for combatting cancer and enhancing treatment efficacy.
Keywords: Pseudomonas aeruginosa; biofilm; ferroptosis; pyoverdine; tumor microenvironment.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., Bray F., Ca‐Cancer J. Clin. 2021, 71, 209. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
