Hibernating ribosomes as drug targets?
- PMID: 39135874
- PMCID: PMC11317432
- DOI: 10.3389/fmicb.2024.1436579
Hibernating ribosomes as drug targets?
Abstract
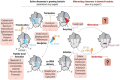
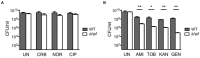
When ribosome-targeting antibiotics attack actively growing bacteria, they occupy ribosomal active centers, causing the ribosomes to stall or make errors that either halt cellular growth or cause bacterial death. However, emerging research indicates that bacterial ribosomes spend a considerable amount of time in an inactive state known as ribosome hibernation, in which they dissociate from their substrates and bind to specialized proteins called ribosome hibernation factors. Since 60% of microbial biomass exists in a dormant state at any given time, these hibernation factors are likely the most common partners of ribosomes in bacterial cells. Furthermore, some hibernation factors occupy ribosomal drug-binding sites - leading to the question of how ribosome hibernation influences antibiotic efficacy, and vice versa. In this review, we summarize the current state of knowledge on physical and functional interactions between hibernation factors and ribosome-targeting antibiotics and explore the possibility of using antibiotics to target not only active but also hibernating ribosomes. Because ribosome hibernation empowers bacteria to withstand harsh conditions such as starvation, stress, and host immunity, this line of research holds promise for medicine, agriculture, and biotechnology: by learning to regulate ribosome hibernation, we could enhance our capacity to manage the survival of microorganisms in dormancy.
Keywords: antimicrobial resistance; dormancy; hibernation; ribosome; ribosome-targeting drugs.
Copyright © 2024 Ekemezie and Melnikov.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Adrian P. V., Mendrick C., Loebenberg D., McNicholas P., Shaw K. J., Klugman K. P., et al. (2000). Evernimicin (SCH27899) inhibits a novel ribosome target site: analysis of 23S ribosomal DNA mutants. Antimicrob. Agents Chemother. 44, 3101–3106. doi: 10.1128/AAC.44.11.3101-3106.2000, PMID: - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

