Ubiquitin-specific protease 22 controls melanoma metastasis and vulnerability to ferroptosis through targeting SIRT1/PTEN/PI3K signaling
- PMID: 39135915
- PMCID: PMC11318338
- DOI: 10.1002/mco2.684
Ubiquitin-specific protease 22 controls melanoma metastasis and vulnerability to ferroptosis through targeting SIRT1/PTEN/PI3K signaling
Abstract
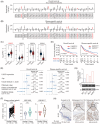
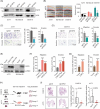
Metastasis is a major contributing factor that affects the prognosis of melanoma patients. Nevertheless, the underlying molecular mechanisms involved in melanoma metastasis are not yet entirely understood. Here, we identified ubiquitin-specific protease 22 (USP22) as a pro-oncogenic protein in melanoma through screening the survival profiles of 52 ubiquitin-specific proteases (USPs). USP22 demonstrates a strong association with poor clinical outcomes and is significantly overexpressed in melanoma. Ablation of USP22 expression remarkably attenuates melanoma migration, invasion, and epithelial-mesenchymal transition in vitro and suppresses melanoma metastasis in vivo. Mechanistically, USP22 controls melanoma metastasis through the SIRT1/PTEN/PI3K pathway. In addition, we conducted an United States Food and Drug Administration-approved drug library screening and identified topotecan as a clinically applicable USP22-targeting molecule by promoting proteasomal degradation of USP22. Finally, we found that both pharmacological and genetic silence of USP22 sensitize RSL3-induced ferroptosis through suppressing the PI3K/Akt/mTOR pathway and its downstream SCD, and ferroptosis inhibitor could partly rescued the decreased lung metastasis by topotecan in vivo. Overall, our findings reveal a prometastatic role of USP22 and identify topotecan as a potent USP22-targeting drug to limit melanoma metastasis.
Keywords: USP22; ferroptosis; melanoma; metastasis; topotecan.
© 2024 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors have no relevant financial or nonfinancial interests to disclose.
Figures





References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: gLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. - PubMed
-
- Millet A, Martin AR, Ronco C, Rocchi S, Benhida R. Metastatic melanoma: insights into the evolution of the treatments and future challenges. Med Res Rev. 2017;37(1):98‐148. - PubMed
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17‐48. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
