Diagnostic Utility of Pre-Genomic Hepatitis B RNA in the Evaluation of HBV/HIV Coinfection
- PMID: 39135958
- PMCID: PMC11318280
- DOI: 10.20411/pai.v9i2.720
Diagnostic Utility of Pre-Genomic Hepatitis B RNA in the Evaluation of HBV/HIV Coinfection
Abstract
Background: Newer biomarkers of Hepatitis B virus (HBV) infection and treatment response have not been well-characterized in individuals with HBV/HIV coinfection.
Methods: Pre-genomic RNA (pgRNA) and quantitative HBsAg (qHBsAg) were used to evaluate the associations with baseline characteristics. Participants included two separate groups - 236 with HBV/HIV coinfection enrolled in a cross-sectional cohort in Ghana and 47 from an HBV nucleoside/nucleotide treatment trial comparing tenofovir to adefovir in the United States.
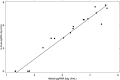
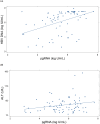
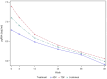
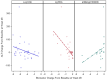
Results: In both cohorts, HBe antigenemia was highly associated with pgRNA and HBV DNA levels. In the treatment cohort, pre-treatment pgRNA serum concentration was 7.0 log10 U/mL, and mean qHBsAg was 201,297 IU/mL. The observed treatment-associated decrease in pgRNA was consistent with a biphasic decline curve that reached second-phase kinetics following treatment week 12. Changes from baseline were significantly correlated with changes in serum ALT (r = - 0.518; P = 0.023) but not with changes in HBV DNA (r = 0.132, P = NS). qHBsAg also correlated with ALT change (r = - 0.488, P = 0.034).
Conclusion: pgRNA and qHBsAg represent newer biomarkers of HBV replication that may help monitor response and treatment outcomes. HBV pgRNA is highly associated with both HBeAg and ALT and may predict both active replication from the closed circular DNA (cccDNA) template as well as hepatic injury.
Keywords: HBV; HBV DNA; HIV; pgRNA; quantitative HBsAg; treatment.
Copyright © 2024 Pathogens and Immunity.
Conflict of interest statement
MA, MS, and GC are employees and shareholders of Abbott Laboratories. All other authors have no conflicts of interest.
Figures
Similar articles
-
HBV pgRNA profiles in Chinese HIV/HBV coinfected patients under pre- and posttreatment: a multicentre observational cohort study.J Viral Hepat. 2022 Aug;29(8):616-626. doi: 10.1111/jvh.13704. Epub 2022 Jun 7. J Viral Hepat. 2022. PMID: 35582838 Free PMC article.
-
Mechanisms downstream of reverse transcription reduce serum levels of HBV DNA but not of HBsAg in chronic hepatitis B virus infection.Virol J. 2015 Dec 9;12:213. doi: 10.1186/s12985-015-0447-5. Virol J. 2015. PMID: 26645241 Free PMC article.
-
Quantitative HBsAg and HBeAg predict hepatitis B seroconversion after initiation of HAART in HIV-HBV coinfected individuals.PLoS One. 2013 Apr 9;8(4):e61297. doi: 10.1371/journal.pone.0061297. Print 2013. PLoS One. 2013. PMID: 23593455 Free PMC article.
-
Progression and status of antiviral monitoring in patients with chronic hepatitis B: From HBsAg to HBV RNA.World J Hepatol. 2018 Sep 27;10(9):603-611. doi: 10.4254/wjh.v10.i9.603. World J Hepatol. 2018. PMID: 30310538 Free PMC article. Review.
-
Antivirals for prevention of hepatitis B virus mother-to-child transmission in human immunodeficiency virus positive pregnant women co-infected with hepatitis B virus.Cochrane Database Syst Rev. 2023 Jun 12;6(6):CD013653. doi: 10.1002/14651858.CD013653.pub2. Cochrane Database Syst Rev. 2023. PMID: 37306558 Free PMC article. Review.
References
-
- Patel EU, Thio CL, Boon D, Thomas DL, Tobian AAR. Prevalence of Hepatitis B and Hepatitis D Virus Infections in the United States, 2011-2016. Clin Infect Dis. 2019;69(4):709–12. doi: 10.1093/cid/ciz001. PubMed PMID: 30605508; PMCID: PMC6669285. - DOI - PMC - PubMed
-
- Sherman KE. Management of the Hepatitis B Virus/HIV-Coinfected Patient. Top Antivir Med. 2015;23(3):111–4. PubMed PMID: 26518394; PMCID: PMC6148934. - PMC - PubMed
-
- Jeng WJ, Lok ASF. What will it take to cure hepatitis B? Hepatol Commun. 2023;7(4). doi: 10.1097/hc9.0000000000000084. PubMed PMID: 36972391; PMCID: PMC10043561. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
