Impact of Antifungal Prophylaxis Continuation or Discontinuation After Allogeneic Hematopoietic Cell Transplant on the Incidence of Invasive Mold Infection
- PMID: 39135965
- PMCID: PMC11317840
- DOI: 10.1093/ofid/ofae409
Impact of Antifungal Prophylaxis Continuation or Discontinuation After Allogeneic Hematopoietic Cell Transplant on the Incidence of Invasive Mold Infection
Abstract
Background: Continuing antifungal prophylaxis (AFPx) to prevent invasive mold infections (IMIs) in recipients of allogeneic hematopoietic cell transplantation (alloHCT) after primary hospital discharge from alloHCT admission varies among transplant centers despite recommendations to continue prophylaxis through day +75. Characteristics driving AFPx prescribing at hospital discharge and outcomes are unknown.
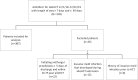
Methods: In this retrospective analysis, we reviewed patients continuing AFPx vs no AFPx at hospital discharge. We included patients with a hospital stay ≥7 days and ≤40 days. We excluded patients with a history of IMI prior to alloHCT, new IMI during admission, or death prior to discharge. Our primary objective was incidence of probable or proven IMI per the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Our secondary objectives were nonrelapse mortality at day +100, overall survival at day +100, and characteristics driving AFPx discontinuation at hospital discharge.
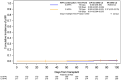
Results: Of the 430 patients identified, 387 met inclusion criteria. At discharge, 56% (217/387) continued AFPx, and 44% (170/387) had no AFPx. At day +100, 3 probable IMI cases occurred in the group with continued AFPx vs 1 probable IMI case in the no-AFPx group (no proven IMI). Univariate analysis showed no difference in cumulative incidence of probable IMI (P = .440), nonrelapse mortality (P = .072), and overall survival (P = .855) between groups. Multivariable logistic regression demonstrated that patients were less likely to continue AFPx if they had a diagnosis other than acute myeloid leukemia, a length of stay ≤30 days, acute graft-vs-host disease grade 0 or 1, and corticosteroid use ≤5 days.
Conclusions: There was no difference in probable IMI at day +100 after alloHCT based on continuing vs discontinuing AFPx at hospital discharge after alloHCT admission supporting a risk-adapted prophylaxis approach.
Keywords: antifungal; hematopoietic; invasive mold infection; prophylaxis; transplant.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. S. J. F. serves as an Science Advisory Board member of Allogene Therapeutics, Lixte Bio Board of Directors, Science Advistory Board, Director, T-Cell Therapeutics Research Laboratories and Hematologic Malignancies Research Institute, City of Hope, Mustang CAR-T cell consultant. R. T. serves as a consultant for Merck and Karius advisory boards. R. N. serves as a consultant for Omeros Corporation, Ono Pharmaceutical, and Pfizer. S. S. D. is a consultant/advisory board member for Merck, Ansun Biopharma, Allovir, Aseptiscope Inc, Takeda, and Karius Research; has received grant support (paid to institution) from Merck, Allovir, Karius, Ansun Biopharma, and F2G; and is on the speakers bureau for Karius, Takeda, and Astellas. All other authors report no potential conflicts.
Figures


References
-
- Slavin MA, Osborne B, Adams R, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation—a prospective, randomized, double-blind study. J Infect Dis 1995; 171:1545–52. - PubMed
-
- van Burik J-AH, Ratanatharathorn V, Stepan DE, et al. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis 2004; 39:1407–16. - PubMed
-
- Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis 2002; 34:909–17. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

