Development of Brain Penetrant Pyridazine Pantothenate Kinase Activators
- PMID: 39136313
- PMCID: PMC11345825
- DOI: 10.1021/acs.jmedchem.4c01211
Development of Brain Penetrant Pyridazine Pantothenate Kinase Activators
Abstract
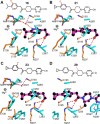
Conversion of pantothenate to phosphopantothenate in humans is the first dedicated step in the coenzyme A (CoA) biosynthesis pathway and is mediated by four isoforms of pantothenate kinase. These enzymes are allosterically regulated by acyl-CoA levels, which control the rate of CoA biosynthesis. Small molecule activators of the PANK enzymes that overcome feedback suppression increase CoA levels in cultured cells and animals and have shown great potential for the treatment of pantothenate kinase-associated neurodegeneration and propionic acidemias. In this study, we detail the further optimization of PANK pyridazine activators using structure-guided design and focus on the cellular CoA activation potential, metabolic stability, and solubility as the primary drivers of the structure-activity relationship. These studies led to the prioritization of three late-stage preclinical lead PANK modulators with improved pharmacokinetic profiles and the ability to substantially increase brain CoA levels. Compound 22 (BBP-671) eventually advanced into clinical testing for the treatment of PKAN and propionic acidemia.
Conflict of interest statement
The authors declare the following competing financial interest(s): The authors disclose ownership of intellectual property rights associated with the technologies discussed in this work.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

