Meflin/ISLR is a marker of adipose stem and progenitor cells in mice and humans that suppresses white adipose tissue remodeling and fibrosis
- PMID: 39136356
- PMCID: PMC11555626
- DOI: 10.1111/gtc.13154
Meflin/ISLR is a marker of adipose stem and progenitor cells in mice and humans that suppresses white adipose tissue remodeling and fibrosis
Abstract
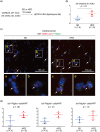
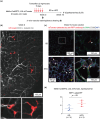
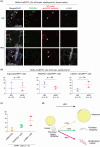
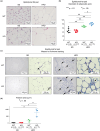
Identifying specific markers of adipose stem and progenitor cells (ASPCs) in vivo is crucial for understanding the biology of white adipose tissues (WAT). PDGFRα-positive perivascular stromal cells represent the best candidates for ASPCs. This cell lineage differentiates into myofibroblasts that contribute to the impairment of WAT function. However, ASPC marker protein(s) that are functionally crucial for maintaining WAT homeostasis are unknown. We previously identified Meflin as a marker of mesenchymal stem cells (MSCs) in bone marrow and tissue-resident perivascular fibroblasts in various tissues. We also demonstrated that Meflin maintains the undifferentiated status of MSCs/fibroblasts. Here, we show that Meflin is expressed in WAT ASPCs. A lineage-tracing experiment showed that Meflin+ ASPCs proliferate in the WAT of obese mice induced by a high-fat diet (HFD), while some of them differentiate into myofibroblasts or mature adipocytes. Meflin knockout mice fed an HFD exhibited a significant fibrotic response as well as increases in adipocyte cell size and the number of crown-like structures in WAT, accompanied by impaired glucose tolerance. These data suggested that Meflin expressed by ASPCs may have a role in reducing disease progression associated with WAT dysfunction.
Keywords: Islr; Meflin; adipose stem and progenitor cells; adipose stem cells; fibrosis; glucose tolerance; immunoglobulin superfamily containing leucine‐rich repeat; mesenchymal stem cell; metabolic syndrome.
© 2024 The Author(s). Genes to Cells published by Molecular Biology Society of Japan and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Ando, R. , Shiraki, Y. , Miyai, Y. , Shimizu, H. , Furuhashi, K. , Minatoguchi, S. , Kato, K. , Kato, A. , Iida, T. , Mizutani, Y. , Ito, K. , Asai, N. , Mii, S. , Esaki, N. , Takahashi, M. , & Enomoto, A. (2024). Meflin is a marker of pancreatic stellate cells involved in fibrosis and epithelial regeneration in the pancreas. The Journal of Pathology, 262(1), 61–75. 10.1002/path.6211 - DOI - PubMed
-
- Buechler, M. B. , Pradhan, R. N. , Krishnamurty, A. T. , Cox, C. , Calviello, A. K. , Wang, A. W. , Yang, Y. A. , Tam, L. , Caothien, R. , Roose‐Girma, M. , Modrusan, Z. , Arron, J. R. , Bourgon, R. , Müller, S. , & Turley, S. J. (2021). Cross‐tissue organization of the fibroblast lineage. Nature, 593(7860), 575–579. 10.1038/s41586-021-03549-5 - DOI - PubMed
-
- Cattaneo, P. , Mukherjee, D. , Spinozzi, S. , Zhang, L. , Larcher, V. , Stallcup, W. B. , Kataoka, H. , Chen, J. , Dimmeler, S. , Evans, S. M. , & Guimarães‐Camboa, N. (2020). Parallel lineage‐tracing studies establish fibroblasts as the prevailing in vivo adipocyte progenitor. Cell Reports, 30(2), 571–582.e2. 10.1016/j.celrep.2019.12.046 - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 22ck0106779h0001/Japan Agency for Medical Research and Development
- 23gm1210009s0105/Japan Agency for Medical Research and Development
- 20H03467/Ministry of Education, Culture, Sports, Science and Technology
- 22H02848/Ministry of Education, Culture, Sports, Science and Technology
- 22K18390/Ministry of Education, Culture, Sports, Science and Technology
LinkOut - more resources
Full Text Sources
Medical

