Ammonium Zincates as Catalysts for the Microwave-Enhanced Synthesis of Symmetric Piperazines by Regioselective Opening of Aziridines
- PMID: 39136397
- PMCID: PMC11581340
- DOI: 10.1002/asia.202400688
Ammonium Zincates as Catalysts for the Microwave-Enhanced Synthesis of Symmetric Piperazines by Regioselective Opening of Aziridines
Abstract
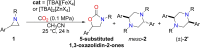
2,5-disubstituted N,N'-alkylpiperazines represent an interesting target in organic synthesis both for pharmaceutical or agrochemical applications and as a promising class of ligands in coordination chemistry. We report here a microwave-enhanced synthesis of these compounds starting from non-activated N-alkyl aziridines in the presence of catalytic amounts of simple ammonium metallates. A remarkable TOF of 2787.9 h-1 has been observed in the case of [TBA]2[ZnI4] as the catalyst (catalyst loading 0.1 mol %) and with an almost complete selectivity (up to 97 %) in favor of both diastereoisomers (meso and chiral form) of the target 2,5-disubstituted piperazines, obtained in 1 : 1 ratio. The two isomers are easily separated, because the meso form precipitates in pure from the reaction crude. A stereochemical investigation and the unprecedented isolation of 2,6-disubstituted N,N'-alkylpiperazines allowed us to shed light on the reaction mechanism.
Keywords: Homogeneous catalysis; Metallates; Microwave chemistry; Piperazine; Zinc.
© 2024 The Authors. Chemistry - An Asian Journal published by Wiley-VCH GmbH.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Sajadikhah S. S., Nassiri M., Chem. Heterocycl. Compd. 2021, 57, 905–907.
-
- Chauhan N., Pradhan S., Ghorai M. K., J. Org. Chem. 2019, 84, 1757–1765. - PubMed
-
- Das B. K., Pradhan S., Punniyamurthy T., Org. Lett. 2018, 20, 4444–4448. - PubMed
-
- Al-Ghorbani M., Bushra Begum A., Zabiulla Z., Mamatha S. V., Khanum S. A., Res. J. Pharm. Technol. 2015, 8, 611–628.
-
- Zhang Y., Liu X., Zou Y., Wang S., Song H., Cai Q., Peng J., Yi C., Chen J., J. Heterocycl. Chem. 2023, 60, 1826–1837.

