In vitro efficacy of next-generation dihydrotriazines and biguanides against babesiosis and malaria parasites
- PMID: 39136469
- PMCID: PMC11373198
- DOI: 10.1128/aac.00423-24
In vitro efficacy of next-generation dihydrotriazines and biguanides against babesiosis and malaria parasites
Abstract
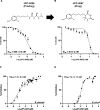
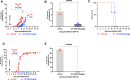
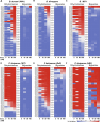
Babesia and Plasmodium pathogens, the causative agents of babesiosis and malaria, are vector-borne intraerythrocytic protozoan parasites, posing significant threats to both human and animal health. The widespread resistance exhibited by these pathogens to various classes of antiparasitic drugs underscores the need for the development of novel and more effective therapeutic strategies. Antifolates have long been recognized as attractive antiparasitic drugs as they target the folate pathway, which is essential for the biosynthesis of purines and pyrimidines, and thus is vital for the survival and proliferation of protozoan parasites. More efficacious and safer analogs within this class are needed to overcome challenges due to resistance to commonly used antifolates, such as pyrimethamine, and to address liabilities associated with the dihydrotriazines, WR99210 and JPC-2067. Here, we utilized an in vitro culture condition suitable for the continuous propagation of Babesia duncani, Babesia divergens, Babesia MO1, and Plasmodium falciparum in human erythrocytes to screen a library of 50 dihydrotriazines and 29 biguanides for their efficacy in vitro and compared their potency and therapeutic indices across different species and isolates. We identified nine analogs that inhibit the growth of all species, including the P. falciparum pyrimethamine-resistant strain HB3, with IC50 values below 10 nM, and display excellent in vitro therapeutic indices. These compounds hold substantial promise as lead antifolates for further development as broad-spectrum antiparasitic drugs.
Keywords: DHFR-TS; antifolates; antiparasitic drugs; apicomplexan; dihydrotriazine; nucleotide biosynthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- World Health O. 2023. World malaria report 2023. World Health Organization, Geneva.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

