Co-existence of two antibiotic-producing marine bacteria: Pseudoalteromonas piscicida reduce gene expression and production of the antibacterial compound, tropodithietic acid, in Phaeobacter sp
- PMID: 39136490
- PMCID: PMC11409694
- DOI: 10.1128/aem.00588-24
Co-existence of two antibiotic-producing marine bacteria: Pseudoalteromonas piscicida reduce gene expression and production of the antibacterial compound, tropodithietic acid, in Phaeobacter sp
Abstract
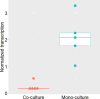
Many bacteria co-exist and produce antibiotics, yet we know little about how they cope and occupy the same niche. The purpose of the present study was to determine if and how two potent antibiotic-producing marine bacteria influence the secondary metabolome of each other. We established an agar- and broth-based system allowing co-existence of a Phaeobacter species and Pseudoalteromonas piscicida that, respectively, produce tropodithietic acid (TDA) and bromoalterochromides (BACs). Co-culturing of Phaeobacter sp. strain A36a-5a on Marine Agar with P. piscicida strain B39bio caused a reduction of TDA production in the Phaeobacter colony. We constructed a transcriptional gene reporter fusion in the tdaC gene in the TDA biosynthetic pathway in Phaeobacter and demonstrated that the reduction of TDA by P. piscicida was due to the suppression of the TDA biosynthesis. A stable liquid co-cultivation system was developed, and the expression of tdaC in Phaeobacter was reduced eightfold lower (per cell) in the co-culture compared to the monoculture. Mass spectrometry imaging of co-cultured colonies revealed a reduction of TDA and indicated that BACs diffused into the Phaeobacter colony. BACs were purified from Pseudoalteromonas; however, when added as pure compounds or a mixture they did not influence TDA production. In co-culture, the metabolome was dominated by Pseudoalteromonas features indicating that production of other Phaeobacter compounds besides TDA was reduced. In conclusion, co-existence of two antibiotic-producing bacteria may be allowed by one causing reduction in the antagonistic potential of the other. The reduction (here of TDA) was not caused by degradation but by a yet uncharacterized mechanism allowing Pseudoalteromonas to reduce expression of the TDA biosynthetic pathway.IMPORTANCEThe drug potential of antimicrobial secondary metabolites has been the main driver of research into these compounds. However, in recent years, their natural role in microbial systems and microbiomes has become important to determine the assembly and development of microbiomes. Herein, we demonstrate that two potent antibiotic-producing bacteria can co-exist, and one mechanism allowing the co-existence is the specific reduction of antibiotic production in one bacterium by the other. Understanding the molecular mechanisms in complex interactions provides insights for applied uses, such as when developing TDA-producing bacteria for use as biocontrol in aquaculture.
Keywords: Phaeobacter; Pseudoalteromonas; bromoalterochromides; mass spectrometry imaging; metabolomics; tropodithietic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

