Quantification of Aortic Valve Fibrotic and Calcific Tissue from CTA: Prospective Comparison with Histology
- PMID: 39136569
- PMCID: PMC11366676
- DOI: 10.1148/radiol.240229
Quantification of Aortic Valve Fibrotic and Calcific Tissue from CTA: Prospective Comparison with Histology
Abstract
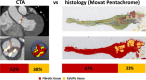
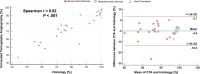
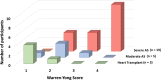
Background Quantifying the fibrotic and calcific composition of the aortic valve at CT angiography (CTA) can be useful for assessing disease severity and outcomes of patients with aortic stenosis (AS); however, it has not yet been validated against quantitative histologic findings. Purpose To compare quantification of aortic valve fibrotic and calcific tissue composition at CTA versus histologic examination. Materials and Methods This prospective study included patients who underwent CTA before either surgical aortic valve replacement for AS or orthotopic heart transplant (controls) at two centers between January 2022 and April 2023. At CTA, fibrotic and calcific tissue composition were quantified using automated Gaussian mixture modeling applied to the density of aortic valve tissue components, calculated as [(volume/total tissue volume) × 100]. For histologic evaluation, explanted valve cusps were stained with Movat pentachrome as well as hematoxylin and eosin. For each cusp, three 5-µm slices were obtained. Fibrotic and calcific tissue composition were quantified using a validated artificial intelligence tool and averaged across the aortic valve. Correlations were assessed using the Spearman rank correlation coefficient. Intermodality and interobserver variability were measured using the intraclass correlation coefficient (ICC) and Bland-Altman plots. Results Twenty-nine participants (mean age, 63 years ± 10 [SD]; 23 male) were evaluated: 19 with severe AS, five with moderate AS, and five controls. Fibrocalcific tissue composition strongly correlated with histologic findings (r = 0.92; P < .001). The agreement between CTA and histologic findings for fibrocalcific tissue quantification was excellent (ICC, 0.94; P = .001), with underestimation of fibrotic composition at CTA (bias, -4.9%; 95% limits of agreement [LoA]: -18.5%, 8.7%). Finally, there was excellent interobserver repeatability for fibrotic (ICC, 0.99) and calcific (ICC, 0.99) aortic valve tissue volume measurements, with no evidence of a difference in measurements between readers (bias, -0.04 cm3 [95% LoA: -0.27 cm3, 0.19 cm3] and 0.02 cm3 [95% LoA: -0.14 cm3, 0.19 cm3], respectively). Conclusion In a direct comparison, standardized quantitative aortic valve tissue characterization at CTA showed excellent concordance with histologic findings and demonstrated interobserver reproducibility. Clinical trial registration no. NCT06136689 Published under a CC BY 4.0 license. Supplemental material is available for this article. See also the editorial by Almeida in this issue.
Conflict of interest statement
Figures



![Bland-Altman plots of interobserver repeatability between two readers
for assessing fibrotic and calcific tissue volume measurements at CT
angiography. No evidence of a difference in measurements was observed
between the two readers for either fibrotic (bias, −0.04 cm3; 95%
limits of agreement [LoA]: −0.27 cm3, 0.19 cm3) or calcific (bias,
0.02 cm3; 95% LoA: −0.14 cm3, 0.19 cm3) tissue volumes.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/dfb4/11366676/5979d71f0840/radiol.240229.fig6.gif)
References
-
- Coffey S , Cox B , Williams MJ . The prevalence, incidence, progression, and risks of aortic valve sclerosis: a systematic review and meta-analysis . J Am Coll Cardiol 2014. ; 63 ( 25 Pt A ): 2852 – 2861 . - PubMed
-
- Pawade T , Clavel MA , Tribouilloy C , et al . Computed tomography aortic valve calcium scoring in patients with aortic stenosis . Circ Cardiovasc Imaging 2018. ; 11 ( 3 ): e007146 . - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

