Novel CDKL5 targets identified in human iPSC-derived neurons
- PMID: 39136782
- PMCID: PMC11335273
- DOI: 10.1007/s00018-024-05389-8
Novel CDKL5 targets identified in human iPSC-derived neurons
Erratum in
-
Correction: Novel CDKL5 targets identified in human iPSC-derived neurons.Cell Mol Life Sci. 2024 Sep 10;81(1):392. doi: 10.1007/s00018-024-05421-x. Cell Mol Life Sci. 2024. PMID: 39254686 Free PMC article. No abstract available.
Abstract
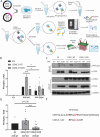
CDKL5 Deficiency Disorder (CDD) is a debilitating epileptic encephalopathy disorder affecting young children with no effective treatments. CDD is caused by pathogenic variants in Cyclin-Dependent Kinase-Like 5 (CDKL5), a protein kinase that regulates key phosphorylation events in neurons. For therapeutic intervention, it is essential to understand molecular pathways and phosphorylation targets of CDKL5. Using an unbiased phosphoproteomic approach we identified novel targets of CDKL5, including GTF2I, PPP1R35, GATAD2A and ZNF219 in human iPSC-derived neuronal cells. The phosphoserine residue in the target proteins lies in the CDKL5 consensus motif. We validated direct phosphorylation of GTF2I and PPP1R35 by CDKL5 using complementary approaches. GTF2I controls axon guidance, cell cycle and neurodevelopment by regulating expression of neuronal genes. PPP1R35 is critical for centriole elongation and cilia morphology, processes that are impaired in CDD. PPP1R35 interacts with CEP131, a known CDKL5 phospho-target. GATAD2A and ZNF219 belong to the Nucleosome Remodelling Deacetylase (NuRD) complex, which regulates neuronal activity-dependent genes and synaptic connectivity. In-depth knowledge of molecular pathways regulated by CDKL5 will allow a better understanding of druggable disease pathways to fast-track therapeutic development.
Keywords: CDKL5 deficiency disorder; GTF2I; Kinase; Neurodevelopmental disorder; PPP1R35; Phosphoproteomics; Phosphorylation.
© 2024. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures





References
MeSH terms
Substances
Supplementary concepts
Grants and funding
- Operational Infrastructure Support Program/State Government of Victoria
- 2018-16/Foundation for Children
- Strategic Pilot Project in Stem Cell/Murdoch Children's Research Institute
- Genomic Medicine Research Grant/Murdoch Children's Research Institute
- Near Miss grant/Murdoch Children's Research Institute
- Strategic Pilot Project in Stem Cell/Murdoch Children's Research Institute
- Genomics Medicine/Murdoch Children's Research Institute
- MDBR-20-106-CDKL5/Orphan Disease Center of the University of Pennsylvania
- CDKL5 Forum Junior Fellowship/Loulou Foundation
- MRF2007465/Medical Research Future Funds (MRFF) Stem Cell Therapies Mission
- Near Miss Pilot Award/Veski
- The Chair in Genomic Medicine/Royal Children's Hospital Foundation
- NNF21CC0073729/Novo Nordisk Foundation reNEW Center for Stem Cell Medicine
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

