Dispersive micro-solid phase extraction based on two MOFs as highly effective adsorbents for analysis of nilotinib in plasma and wastewater
- PMID: 39136927
- PMCID: PMC11555170
- DOI: 10.1007/s40199-024-00531-0
Dispersive micro-solid phase extraction based on two MOFs as highly effective adsorbents for analysis of nilotinib in plasma and wastewater
Abstract
Background: Nilotinib (NIL) is a prescription medication employed in the treatment of specific types of leukemia, namely chronic myelogenous leukemia (CML). The determination of NIL levels in patients undergoing treatment for CML is of paramount importance for effective management of treatment and toxicity. Also, monitoring and controlling its level in wastewater sources could help scientists to identify potential hotspots of contamination and take appropriate measures to mitigate their impact on the environment and public health.
Objectives: This study presents a D-µ-SPE technique utilizing two MOFs as adsorbents for the efficient detection of nilotinib in plasma and wastewater samples for the first time.
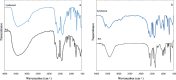
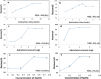
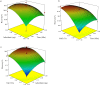
Methods: Two highly effective MOFs, MIL-101(Fe) and MIL-53(Al), were synthesized and applied as dispersive micro-solid phase extraction (D-µ-SPE) adsorbents for the extraction of nilotinib coupled with HPLC-UV in a short time of analysis. Experimental parameters affecting extraction efficacy such as adsorbent amount, ionic strength, pH value, adsorption-desorption time and type of elution solvent, were optimized.
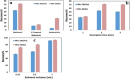
Results: Under optimal experimental conditions, the linear dynamic was achieved in the range of 0.25-5.00 µg/mL in human plasma and 0.01-0.20 µg/mL in wastewater. The extraction recovery was in the range of 89.18-91.53% and 94.39-99.60% for nilotinib and MIL-101(Fe) and also 91.22-97.35% and 98.14-100.78% for nilotinib and MIL-53(Al) from human plasma and wastewater respectively.
Conclusion: HPLC-UV determination of nilotinib after the D-µ-SPE method showed acceptable accuracy and precision in both plasma and wastewater. In comparison between the two adsorbents, the extraction procedure was easier and faster with MIL-53(Al) as the adsorbent.
Keywords: Dispersive micro-solid phase extraction; High performance liquid chromatography-ultra violet spectroscopy; MIL101(Fe); MIL53(Al); Nilotinib.
© 2024. The Author(s), under exclusive licence to Tehran University of Medical Sciences.
Conflict of interest statement
Figures





References
-
- Nakamura R, Fujii H, Yamada T, Matsui Y, Yaoi T, Honda M, Tanaka N, Miyagawa-Hayashino A, Yoshimura A, Morimoto K, Iwasaku M, Tokuda S, Kim YH, Konishi E, Itoh K, Takayama K. Analysis of Tumor Heterogeneity Through AXL Activation in Primary Resistance to EGFR Tyrosine Kinase Inhibitors. JTO Clin Res Repo. 2023;4(6):100525. 10.1016/j.jtocrr.2023.100525. - DOI - PMC - PubMed
-
- Šalamúnová P, Krejčí T, Ryšánek P, Saloň I, Kroupová J, Hubatová-Vacková A, Petřík J, Grus T, Lukáč P, Kozlík P, Křížek T, Dammer O, Beránek J, Šíma M, Slanař O, Štěpánek F. Serum and lymph pharmacokinetics of nilotinib delivered by yeast glucan particles per os. Int J Pharmaceutics. 2023;634:122627. 10.1016/j.ijpharm.2023.122627. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

