Patient-Reported Outcome Measures in Cancer Care: An Updated Systematic Review and Meta-Analysis
- PMID: 39136947
- PMCID: PMC11322847
- DOI: 10.1001/jamanetworkopen.2024.24793
Patient-Reported Outcome Measures in Cancer Care: An Updated Systematic Review and Meta-Analysis
Abstract
Importance: Patient-reported outcome measures (PROMs) come directly from the patient, without clinician interpretation, to provide a patient-centered perspective.
Objective: To understand the association of PROM integration into cancer care with patient-related, therapy-related, and health care utilization outcomes.
Data sources: Searches included MEDLINE and MEDLINE Epub ahead of print, in-process, and other nonindexed citations; Embase databases (OvidSP); PsychINFO; CENTRAL; and CINAHL from January 1, 2012 to September 26, 2022.
Study selection: Randomized clinical trials (RCTs) that enrolled adult patients (ages 18 years and older) with active cancer receiving anticancer therapy using a PROM as an intervention.
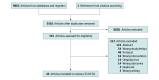
Data extraction and synthesis: Pairs of review authors, using prepiloted forms, independently extracted trial characteristics, disease characteristics, and intervention details. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses reporting guideline was followed. Random-effects analyses were conducted.
Main outcomes and measures: Overall mortality, health-related quality of life (HRQoL) measures, and hospital utilization outcomes.
Results: From 1996 to 2022, 45 RCTs including 13 661 participants addressed the association of PROMs with outcomes considered important to patients. The addition of a PROM likely reduced the risk of overall mortality (HR, 0.84; 95% CI, 0.72-0.98; moderate certainty), improved HRQoL (range 0-100) at 12 weeks (mean difference [MD], 2.45; 95% CI, 0.42-4.48; moderate certainty). Improvements of HRQoL at 24 weeks were not significant (MD, 1.87; 95% CI, -1.21 to 4.96; low certainty). There was no association between the addition of a PROM and HRQoL at 48 weeks. The addition of a PROM was not associated with reduced ED visits (OR, 0.74; 95% CI, 0.54-1.02; low certainty) or hospital admissions (OR, 0.86; 95% CI, 0.73-1.02; low certainty).
Conclusion and relevance: The findings of this study suggest that the integration of PROMs into cancer care may improve overall survival and quality of life.
Conflict of interest statement
Figures
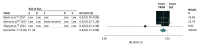


Comment in
-
Patient-Reported Outcome Measures Help Patients With Cancer.JAMA Netw Open. 2024 Aug 1;7(8):e2424748. doi: 10.1001/jamanetworkopen.2024.24748. JAMA Netw Open. 2024. PMID: 39136952 No abstract available.
References
-
- Atkinson TM, Andreotti CF, Roberts KE, Saracino RM, Hernandez M, Basch E. The level of association between functional performance status measures and patient-reported outcomes in cancer patients: a systematic review. Support Care Cancer. 2015;23(12):3645-3652. doi: 10.1007/s00520-015-2923-2 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

