Teplizumab induces persistent changes in the antigen-specific repertoire in individuals at risk for type 1 diabetes
- PMID: 39137044
- PMCID: PMC11405034
- DOI: 10.1172/JCI177492
Teplizumab induces persistent changes in the antigen-specific repertoire in individuals at risk for type 1 diabetes
Abstract
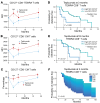
BACKGROUNDTeplizumab, a non-FcR-binding anti-CD3 mAb, is approved to delay progression of type 1 diabetes (T1D) in at-risk patients. Previous investigations described the immediate effects of the 14-day treatment, but longer-term effects of the drug remain unknown.METHODSWith an extended analysis of study participants, we found that 36% were undiagnosed or remained free of clinical diabetes after 5 years, suggesting operational tolerance. Using single-cell RNA sequencing, we compared the phenotypes, transcriptome, and repertoire of peripheral blood CD8+ T cells including autoreactive T cells from study participants before and after teplizumab and features of responders and non-responders.RESULTSAt 3 months, there were transcriptional signatures of cell activation in CD4+ and CD8+ T cells including signaling that was reversed at 18 months. At that time, there was reduced expression of genes in T cell receptor and activation pathways in clinical responders. In CD8+ T cells, we found increased expression of genes associated with exhaustion and immune regulation with teplizumab treatment. These transcriptional features were further confirmed in an independent cohort. Pseudotime analysis showed differentiation of CD8+ exhausted and memory cells with teplizumab treatment. IL7R expression was reduced, and patients with lower expression of CD127 had longer diabetes-free intervals. In addition, the frequency of autoantigen-reactive CD8+ T cells, which expanded in the placebo group over 18 months, did not increase in the teplizumab group.CONCLUSIONThese findings indicate that teplizumab promotes operational tolerance in T1D, involving activation followed by exhaustion and regulation, and prevents expansion of autoreactive T cells.TRIAL REGISTRATIONClinicalTrials.gov NCT01030861.FUNDINGNational Institute of Diabetes and Digestive and Kidney Diseases/NIH, Juvenile Diabetes Research Foundation.
Keywords: Adaptive immunity; Autoimmunity; Endocrinology; T cells; Tolerance.
Figures







References
-
- Herold KC, et al. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013;62(11):3766–3774. doi: 10.2337/db13-0345. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 DK085476/DK/NIDDK NIH HHS/United States
- R01 DK129523/DK/NIDDK NIH HHS/United States
- U01 DK061010/DK/NIDDK NIH HHS/United States
- U01 DK085499/DK/NIDDK NIH HHS/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
- U01 DK061042/DK/NIDDK NIH HHS/United States
- U01 DK085466/DK/NIDDK NIH HHS/United States
- U01 DK103153/DK/NIDDK NIH HHS/United States
- U01 DK061058/DK/NIDDK NIH HHS/United States
- R21 AI166387/AI/NIAID NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- U01 DK085453/DK/NIDDK NIH HHS/United States
- U01 DK106993/DK/NIDDK NIH HHS/United States
- R01 DK057846/DK/NIDDK NIH HHS/United States
- U01 DK061034/DK/NIDDK NIH HHS/United States
- U01 DK085461/DK/NIDDK NIH HHS/United States
- U01 DK085509/DK/NIDDK NIH HHS/United States
- S10 OD030363/OD/NIH HHS/United States
- U01 DK103180/DK/NIDDK NIH HHS/United States
- U01 DK085465/DK/NIDDK NIH HHS/United States
- U01 DK085504/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

